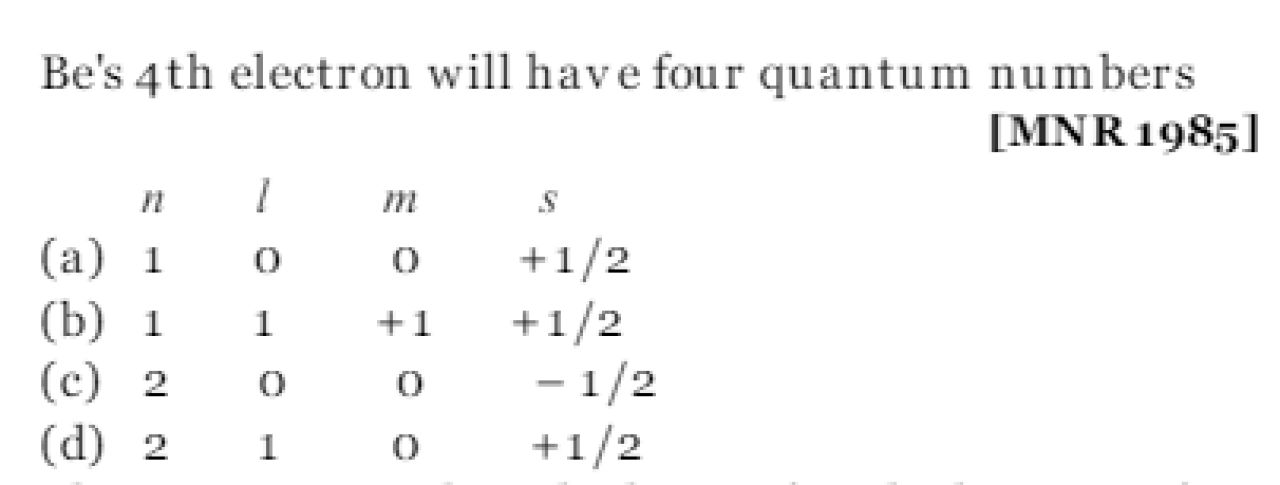Home/Atomic Strcuture
Electronic configuration
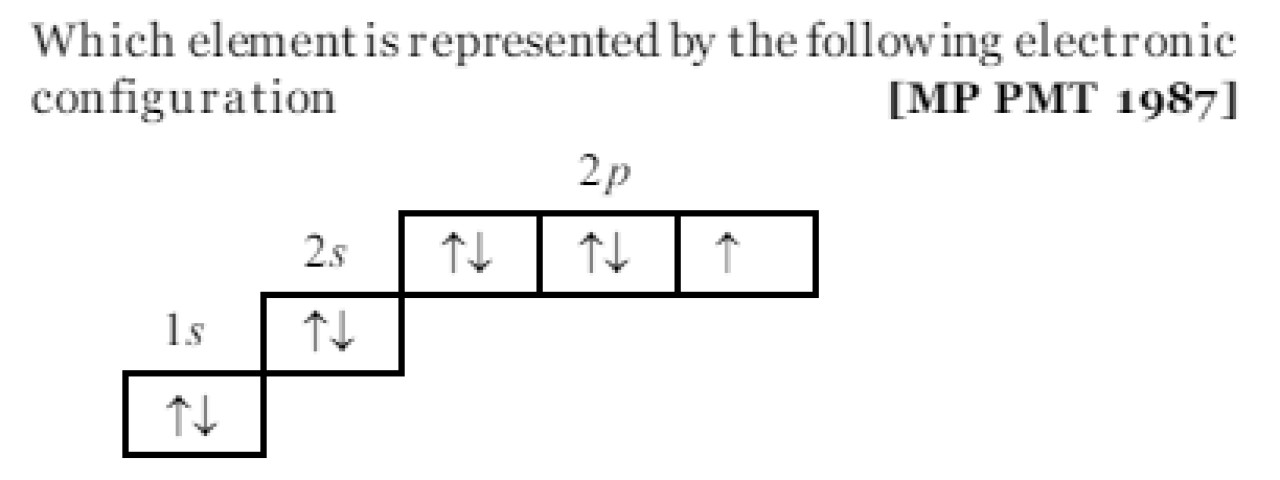
Question#1
Which of the following electronic configuration is correct for carbon? MDCAT-2024
Question#2
Question#3
Question#4
Which one is the electronic configuration of Fe+2 [MA DT Bihar 1982; AIIMS 1989]
Question#5
Cu2+ will have the following electronic configuration [MP PMT 1985]
Question#6
Fe (atomic number = 26) atom has the electronic arrangement [NCERT 1974; MNR 1980]
Question#7
Which of the following represents the electronic configuration of an element with atomic number 17 [AMU 1982]
Question#8
The electronic configuration of an element with atomic number 7 i.e. nitrogen atom is [CPMT 1982, 84, 87]
Question#9
Elements upto atomic number 103 have been synthesized and studied. If a newly discovered element is found to have an atomic number 106, its electronic configuration will be [A IIMS 1980]
Question#10
The electronic configuration of copper ( 29Cu ) is [DPMT 1983; BHU 1980; AFMC 1981; CBSE PMT 1991; MP PMT 1995]
Question#11
The electronic configuration of calcium ion ( Ca2+ ) is [CMC Vellore 1991]
Question#12
Question#13
An element has the electronic configuration 1s2,2s2 2p6,3s2 3p2. Its valency electrons are [NCERT 1973]
Question#14
The electronic configuration of H- is [CPMT 1985]
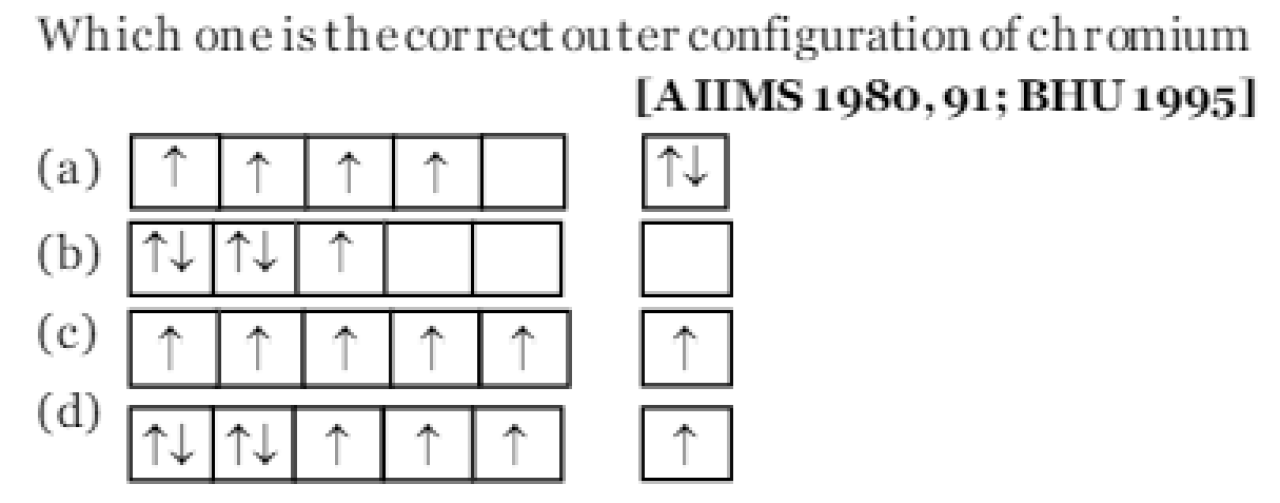
Question#15
The correct ground state electronic configuration of chromium atom is [IIT 1989, 94; MP PMT 1993; EA MCET 1997;ISM Dh anbad 1994; AFMC 1997; Bihar MEE 1996; MP PET 1995, 97; CPMT 1999; Kerala PMT 2003]
Question#16
The electronic configuration of silver atom in ground state is [CPMT 1984, 93]
Question#17
Which one of the following configuration represents a noble gas [CPMT 1983, 89, 93; NCERT 1973; MP PMT 1989; DPMT 1984]
Question#18
Question#19
Which of the following electronic configuration is correct for Cr24 MDCAT2023
Question#20
What is the proton number (atomic number) of an element that has four unpaired electrons in its ground state? MDCAT2023
Question#21
Which element has the ground state electronic configuration of \( 1s^2, 2s^2,2p^6,3s^2, 3p^6 \) MDCAT2023
Question#22
Electronic configuration of deuterium atom is [J&K CET 2005]
Question#23
Electronic configuration 1s2 , 2s2 2p6 , 3s2 3p6 3d5 ,4s1 represents [CPMT 2003]
Question#24
1s2, 2s2, 2p6, 3s1 shows configuration of [CPMT 1996]
Question#25
Which of following pair has the same electronic configuration as possessed by Neon-10 MDCAT2015
Question#26
Which of following electronic configuration is/are correct? ETEA-2016
| i) | 29Cu =1s2 2s2 2p6 3s1 |
| ii) | 29Cu =[Ar] 4s1,3d10 |
| iii) | 24Cr= [Ar] 4s1,3d10 |
| iv | 24Cr= [Ar] 4s2,3d4 |
Question#27
The electronic configuration of a di-valent metal M+ is 2,8,14 its atomic weigh is 56a.m.u the number of neutron in its nuclei would be MNR-1984,89
Question#28
Max number of electrons in electronic configuration can be calculated by using formula MDCAT2016
Question#29
Copper is an typical transition element. its atomic mass is 29. in which oxidation state does it have partially filled orbitals in d-subshell MDCAT2019
Question#30
The correct ground state electronic configuration of Cr MDCAT2019 [IIT 1989, 94; MP PMT 1993; EA MCET 1997;ISM Dh anbad 1994; AFMC 1997; Bihar MEE 1996; MP PET 1995, 97; CPMT 1999; Kerala PMT 2003]
Question#31
What is the proton(atomic number) of an element that has four unpaired electrons in its ground state MDCAT2022
Question#32
Which element has the ground state electronic configuration of 1s2,2s2,2p6,3s2,3p6 MDCAT2022
Question#33
Which pair has 1 electron in its ''s'' orbital? MdCAT(2020)