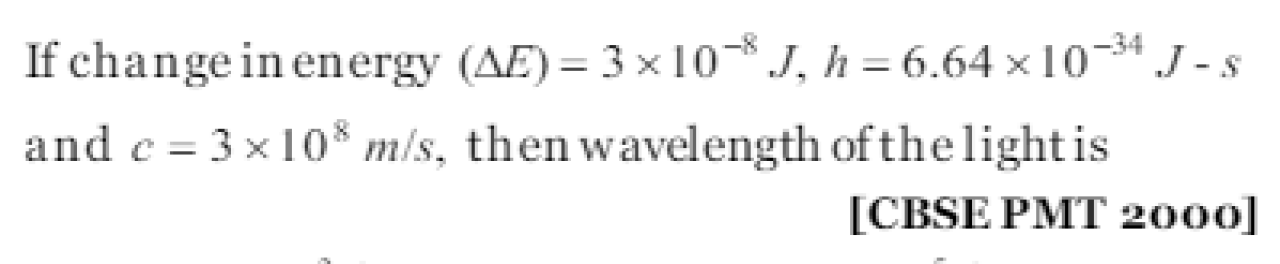Home/Atomic Strcuture
Planks Theory
Question#1
In which one of the following pairs of experimental observations and phenomenon does the experimental observation correctly account for the phenomenon? Experimental Observation Phenomenon [A IIMS 1983]
Question#2
A metal surface is exposed to solar radiations [DPMT 2005]
Question#3
Time taken for an electron to complete one revolution in the Bohr orbit of hydrogen atom is [Kera la PMT 2004]
Question#4
The radius of electron in the first excited state of hydrogen atom is [MP PMT 2004]
Question#5
The mass of a photon with a wavelength equal to cm 1.54 x 10-8 is [Pb. PMT 2004]
Question#6
The frequency of yellow light having wavelength 600 nm is [MP PET 2002]
Question#7
The energy of a radiation of wavelength 8000 Å is E1 and energy of a radiation of wavelength 16000 Å is E2 . What is the relation between these two [Kerala CET 2005]
Question#8
Wavelength of spectral line emitted is inversely proportional to
Question#9
Question#10
Energy of electron of hydrogen atom in second Bohr orbit is [MP PMT 2000]
Question#11
The energy of a photon is calculated by [Pb. PMT 2000]
Question#12
Which one of the following is considered as the main postulate of Bohr’s model of atom [A MU 2000]
Question#13
In hydrogen spectrum the different lines of Ly man series are present is [UPSEA T 1999]
Question#14
Wavelength associated with electron motion [BHU 1998]
Question#15
What is the packet of energy called [AFMC 2005]
Question#16
The wav elength of a spectral line for an electronic transition is inversely related to [IIT 1988]
Question#17
Which one of the following is not the characteristic of Planck's quantum theory of radiation [AIIMS 1991]
Question#18
The ratio of energy of photon of 2000angstrom wavelength to that of 4000angstrom radiation is IIT1986 DCE2000 JIPMER2000
Question#19
The energy of a radiation of wavelength 8000angstrom is E1 and energy of a radiation of wavelength 16000angstrom is E2. What is the relation between these two Kerala CET-2005
Question#20
If change in energy (dE =3 x 10-8 h), h=6.64 x 10-34 Js , c=3 x 108ms-1 then wavelength of the light is CBSE PMT 2000
Question#21
A particular station of India Radio, New Delhi broadcast on a frequency of 1,368KHz. The wavelength of the electromagnetic radiation emitted by the transmitter is NEET-2021