Home/s and p block Elements
Periodic properties (Atomic radius, I.E, E.A, E.N, MP, B.P, O.S, Electrical conductance, Hydration energy )
Question#1
Question#2
Which increases down the group for Alkali metals NUMS2021
Question#3
If first ionization enthalpies of element X and Y are 419KJmol-1 and 590KJmol-1 respectively and second ionization energies of X and Y are 3060KJmol-1 and 1145KJmol-1 respectively re-NEET2022
the correct statement is
Question#4
Ionization energy decreases down the group from top to bottom due to MDCAT2019
Question#5
Oxidation number of particular element can be directly or indirectly inferred from its: MDCAT2019
Question#6
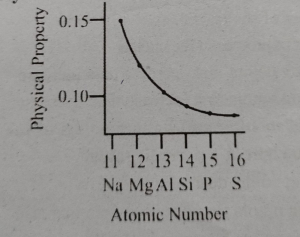
The following sketch show the variation in a physical property of third period elements against their number MDCAT2018
What physical property is plotted in this sketch ?
Question#7
In the period 2 and period 3 maximum melting point shown by elements MDCAT2018
Question#8
Down the group acid-base behavior of metallic oxide of the group 2 elements changes to MDCAT2018
Question#9
Ionic radius along the period is decreases due to MDCAT2017
Question#10
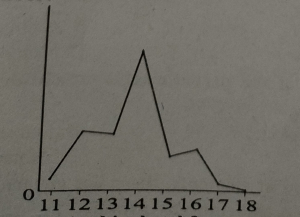
The following sketch shows the melting point of eight elements with consecutive atomic number numbers. Which element is silicon? MDCAT2017
Question#11
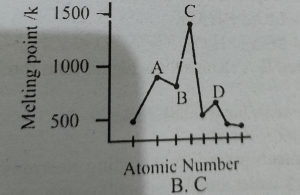
Following graph shows a physical property along the period 3 elements. which physical property is MDCAT2017
Question#12
The ionic radius of the fluoride ion is MDCAT2016
Question#13
Which one of the following will have the smallest radius ? MDCAT2015
Question#14
Keeping in view the size of atoms, which order is correct? MDCAT2015
Question#15
The trend in melting points of the elements of 3rd period are depicted in figure below MDCAT2014
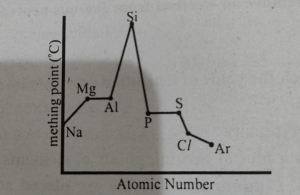
The sharp decrease from 'Si'' to ''P'' is due to
Question#16
Arrange the following elements according to the trend of ionization energies C, N, Ne and B MDCAT2014
Question#17
What is the trend of melting and boiling points of the elements of short period as we move from left to right in a periodic table MDCAT2013
Question#18
Along a period atomic radius decreases. This gradual decrease in radius is due to MDCAT2013
Question#19
More ionization energy of an element MDCAT2012
\( I.E \propto \frac{1}{Metallic \ character} \)
Question#20
Question#21
The element for which the value of ionization energy is low can MDCAT2011
Question#22
Energy required to remove an electron from gaseous neutral atom is MDCAT2010