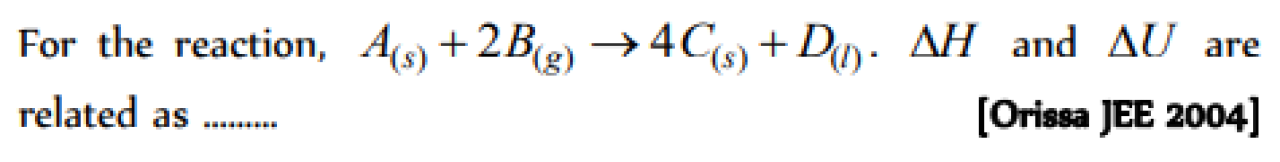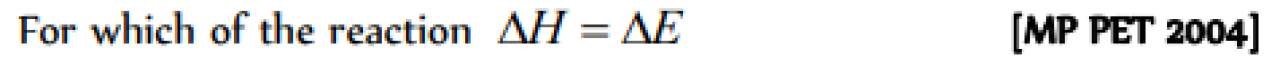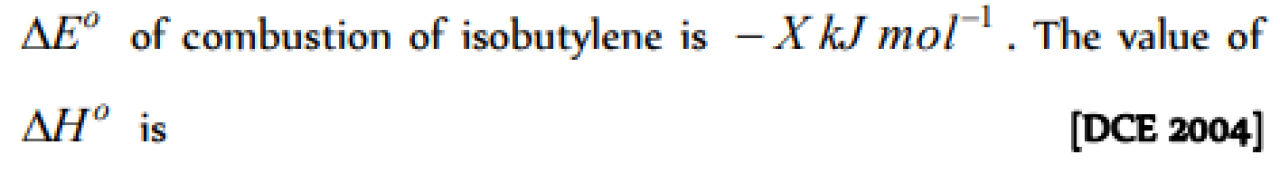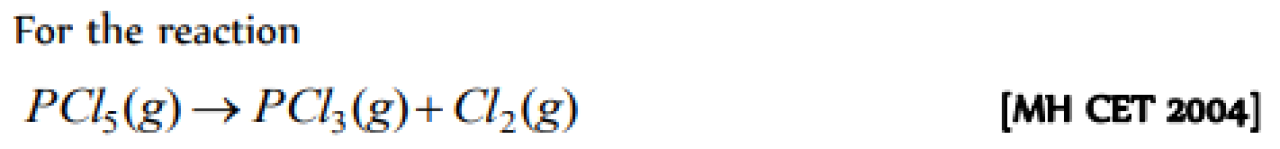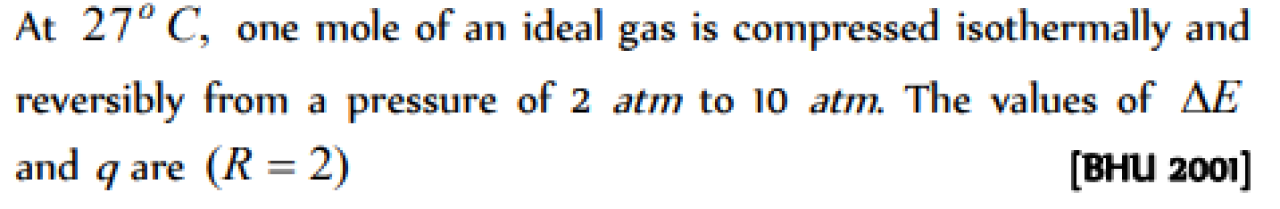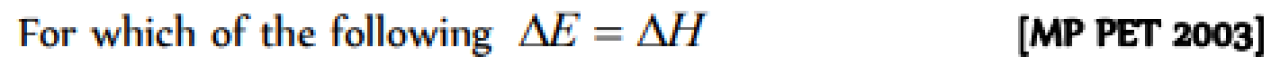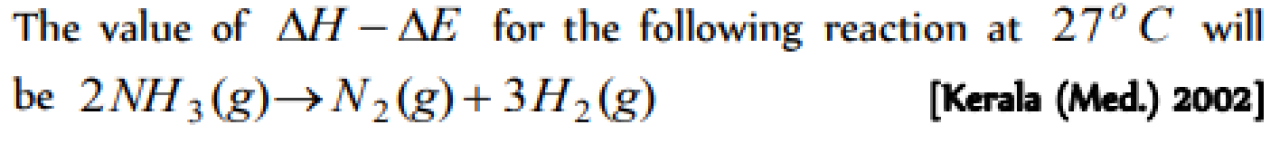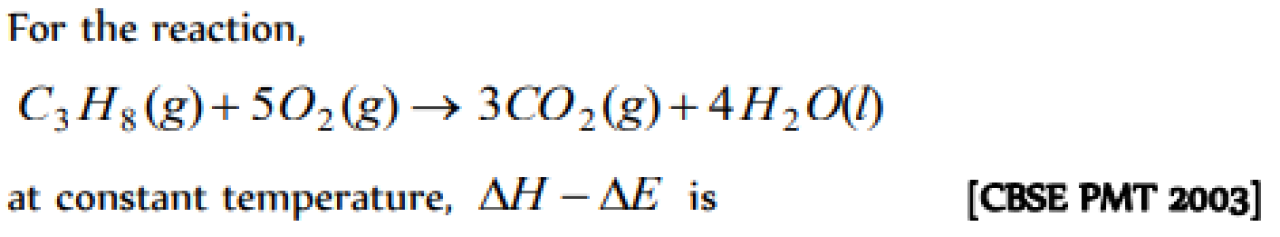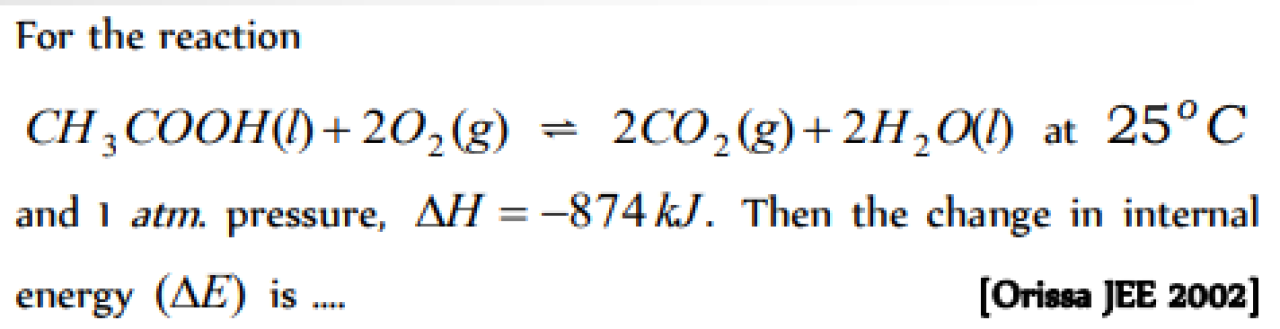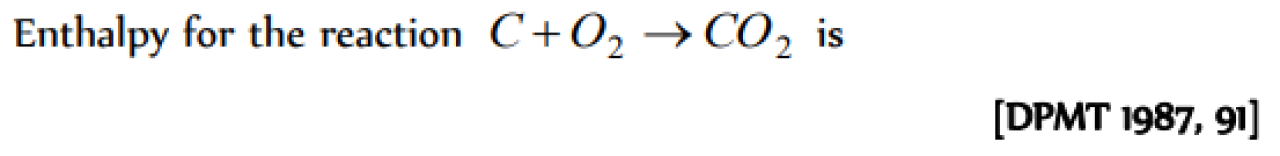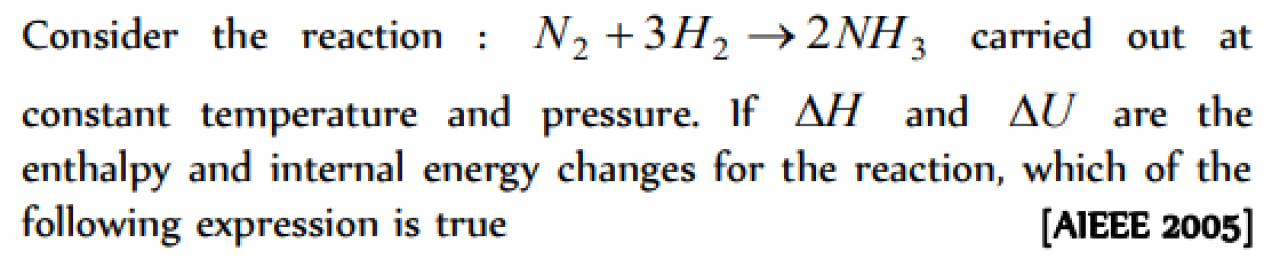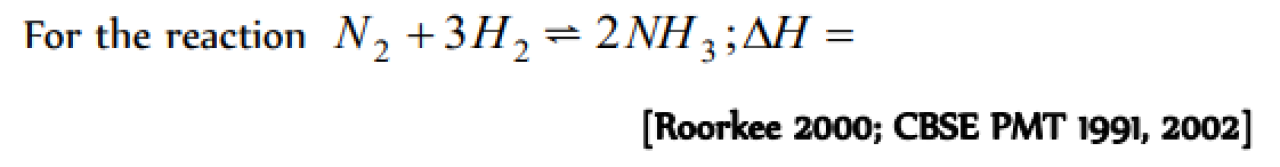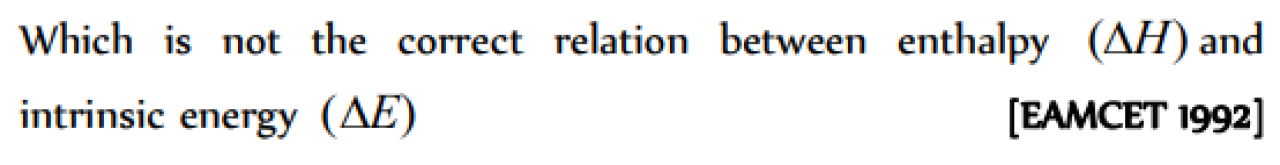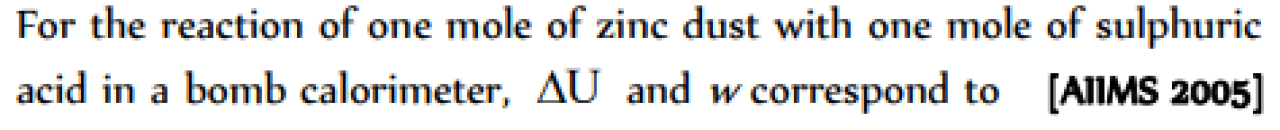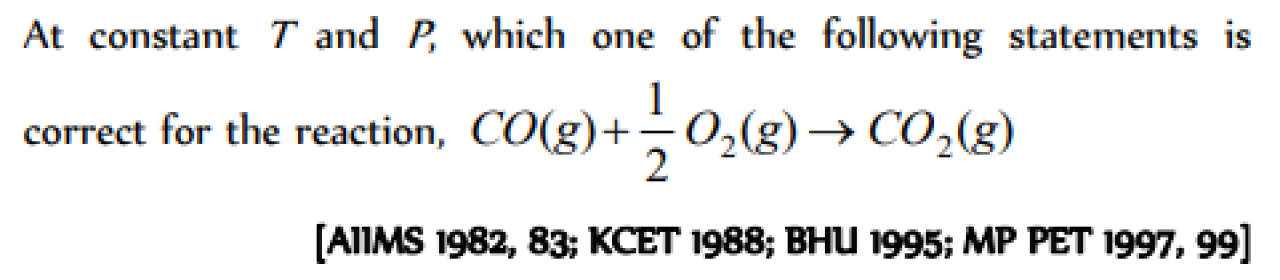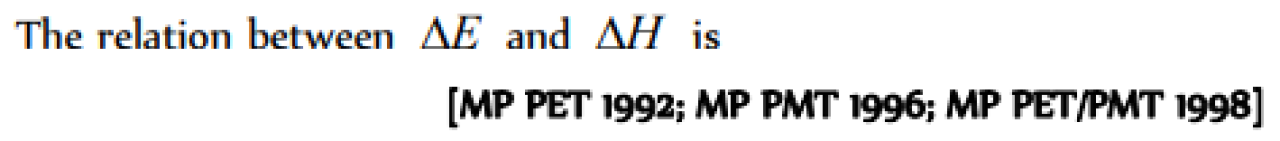Home/Thermochemistry
First Law of thermodynamics
Question#1
Question#2
The work done during the expansion of a gas from a volume of 4dm3 to 6dm3 against a constant external pressure of 3atm is ( 1L atm = 101.32 J ) [CBSE PMT 2004]
Question#3
Question#4
Internal energy is [AFMC 2004]
Question#5
Question#6
Question#7
Question#8
Which of the following is correct regarding the internal energy of a substance [Pb. CET 2002]
Question#9
One mole of an ideal gas is allowed to expand reversibly and adibatically from a temperature of 27oC . If the work done during the process is 3 kJ , then final temperature of the gas is (CV =20 J / K) [Pb. CET 2002]
Question#10
Question#11
Enthalpy (H) is equal to [MH CET 2004]
Question#12
Question#13
If gas, at constant temperature and pressure expands then its [MH CET 2003]
Question#14
Work done during isothermal expansion of one mole of an ideal gas from 10 atom. to 1 atm at 300K is [BHU 2004]
Question#15
A system absorb 600J of heat and work equivalent to 300J on its surroundings. The change in internal energy is [Pb. PMT 2004]
Question#16
The work done by a system is 8 joule, when 40 joule heat is supplied to it. What is the increase in internal energy of system [BHU 2001]
Question#17
Question#18
One mole of an ideal gas is allowed to expand freely and adiabatically into vacuum until its volume has doubled. A statement which is not true concerning this expression is [Pb. PMT 1998]
Question#19
Question#20
Question#21
According to Hess’s law, the heat of reaction depends upon [MP PMT 2003]
Question#22
Question#23
Hess law is based on [MH CET 2002]
Question#24
In a closed insulated container, a liquid is stirred with a paddle to increase its temperature. In this process, which of the following is true [CBSE PMT 2002]
Question#25
Question#26
In an adiabatic expansion of an ideal gas [KCET (Med.) 2001; MH CET 2000]
Question#27
Question#28
Joule-Thomson expansion is [JIPMER 2000]
Question#29
Work done during isothermal expansion of one mole of an ideal gas from 10 atm to 1 atm at 300 K is (Gas constant = 2) [AIIMS 2000]
Question#30
The enthalpy of neutralization of which of the following acids and bases is nearly -13.6 Kcal [Roorkee 1999]
Question#31
Question#32
The internal energy of a substance [KCET 1998; AFMC 2001; AIIMS 2001]
Question#33
An ideal gas at constant temperature and pressure expands, then its [BHU 1998]
Question#34
Hess law of heat summation includes [AFMC 1992]
Question#35
Question#36
In a reversible isothermal process, the change in internal energy is
Question#37
Which of the following expressions represents the first law of thermodynamics [MP PET 1996, 2000; AFMC 1997; BHU 1999; AMU 2000; KCET (Med.) 2000, 01; CBSE PMT 2000; MP PMT 2002]
Question#38
The heat Q for a reaction at constant volume is equal to
Question#39
The enthalpies of the elements in their standard states are assumed to be
Question#40
The law of conservation of energy states that [NCERT 1984]
Question#41
The work done in ergs for the reversible expansion of one mole of an ideal gas from a volume of 10 liters to 20 litres at 25oC is [CMC Vellore 1999]
Question#42
. During an isothermal expansion of an ideal gas its [CBSE PMT 1991]
Question#43
Question#44
Hess law is applicable for the determination of heat of [AIIMS 1998; Pb. PET/PMT 1999]
Question#45
Question#46
Question#47
Hess's law of constant heat summation in based on [MP PET 2001]
Question#48
“The resultant heat change in a reaction is the same whether it takes place in one or several stages.” This statement is called [MP PMT/PET 1988; MP PMT 1989]
Question#49
Question#50
Question#51
The law of Lavoisier and Laplace illustrates [KCET 1989]
Question#52
Question#53
Question#54
Question#55
Question#56
Which of the following is always negative for exothermic reaction?[BCECE 2005]
Question#57
Question#58
The first law of thermodynamics is only
Question#59
The thermal energy at constant pressure is called MCDAT2020
Question#60
An ideal gas at constant temperature and pressure expands, then its [BHU 1998]
Question#61
Which of the following expressions represents the first law of thermodynamics
[MP PET 1996, 2000; AFMC 1997; BHU 1999; AMU 2000;
KCET (Med.) 2000, 01; CBSE PMT 2000; MP PMT 2002]
Question#62
During an isothermal expansion of an ideal gas its
[CBSE PMT 1991]
Question#63
If ΔH is the change in enthalpy and ΔE the change in internal energy accompanying a gaseous reaction
[KCET 1989; CBSE PMT 1990]
Question#64
For the reaction
N2 + 3H2 ? 2NH3 ;ΔH= ? [Roorkee 2000; CBSE PMT 1991, 2002]
Question#65
Which is not the correct relation between enthalpy (ΔH) and intrinsic energy (ΔE)
[EAMCET 1992]