Question#1
The compound not soluble in acetic acid is [UPSEAT 2003; IIT-JEE 1986]
CaC2O4 is a salt of oxalic acid which is more acidic than acetic acid, so it is insoluble in acetic acid.
Question#2
Identify the correct order of boiling points of the following compounds
(1) CH3CH2CH2CH2OH ,
(2) CH3CH2CH2CHO ,
(3) CH3CH2CH2COOH [IIT-JEE (Screening) 2002]
–COOH and –OH group form the hydrogen bond by which they have high boiling point. –COOH group show strong hydrogen bonding so it form dimer and have more boiling point than –OH group. While –CHO group do not form hydrogen bond. Thus the reactivity order are as 3 > 1 > 2.
Question#3
Fruity smell is given by [MH CET 2004]
Question#4
What is the % of acetic acid present in vinegar? [AFMC – 2004; MH CET 2003; CPMT 1974, 75]
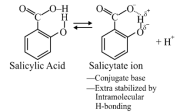
Question#5
Benzoic acid is less acidic than salicylic acid because of [Bihar MEE 1997]
Question#6
Lower carboxylic acids are soluble in water due to [MP PET 1999]
Question#7
Given below are some statements concerning formic acid, which of them is true [CPMT 1983]
Question#8
Formic acid [MP PET/PMT 1988]
Question#9
Acetic acid dissolved in benzene shows a molecular mass of [MP PET 1993]
Question#10
Acetic acid exists as a dimer in benzene solution. This is due to [MP PMT 1989; CPMT 1982]
Question#11
When carboxylic acid and dicarboxylic acids have similar molecular weights how do their melting points compare MDCAT2022
Question#12
When carboxylic acid and dicarboxylic acids have similar molecular weights how do their melting points compare MDCAT2022
Question#13
Which one of the following exist in the form of cyclic dimer ? SET2019
Question#14
Carboxylic acids are soluble is water due to NUMS2021