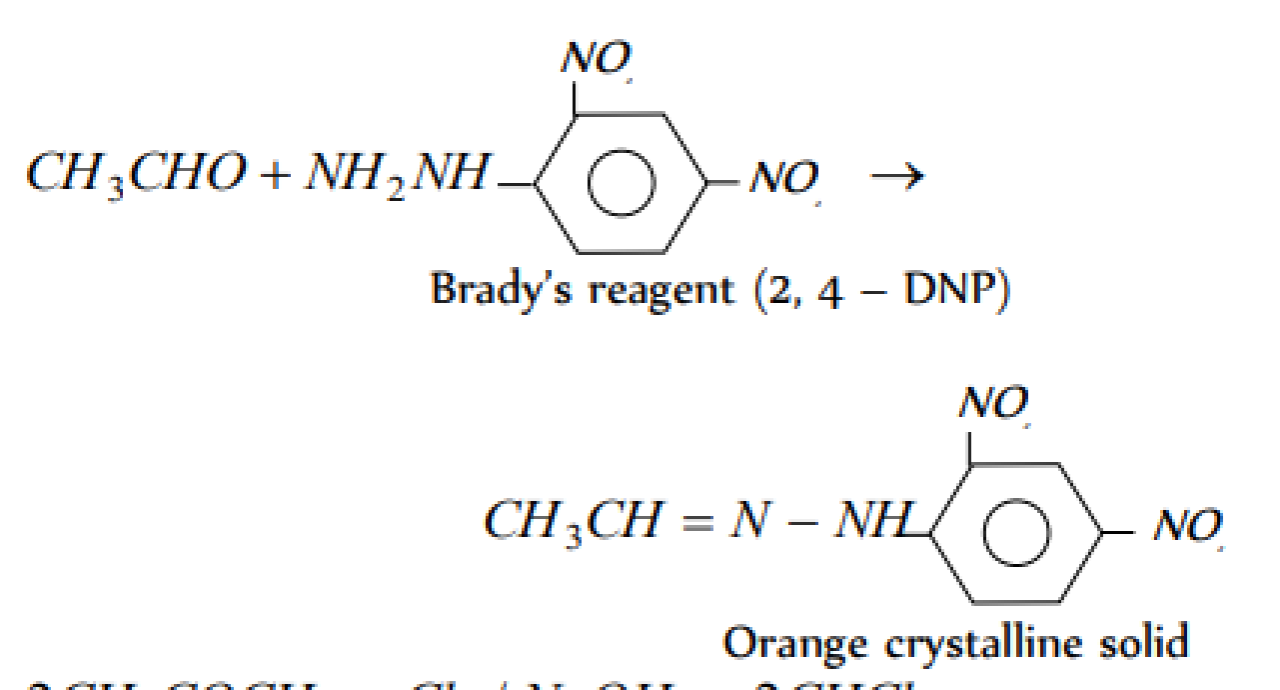Home/Aldehyde and ketones
Identifications Reactions
Question#1
If phenol is treated with 3 moles of concentrated HNO3 in the presence of H2SO4 what the product will be the product? MDCAT2023
Question#2
Appearance of a silver mirror in tollens test indicates the presence of which of the following.? MDCAT2023
Question#3
The compound which reacts with Fehling solution is [CPMT 1989]
Question#4
\( Glucose + Tollen's reagent \rightarrow Silver mirror \) shows [CPMT 1997]
Question#5
When acetaldehyde is heated with Tollen's reagent, following is obtained [CPMT 1989; MP PET/PMT 1988]
Question#6
Which gives difference between aldehyde and ketone [CPMT 1994]
Question#7
Which one is used in the manufacture of mirror [MP PET 1992]
Question#8
The reagent that gives an orange coloured precipitate with acetaldehyde [EAMCET 1997; Pb. PMT 2004; AIIMS 1987]
Question#9
Acetaldehyde and acetone can be distinguished by [AIIMS 1996; DCE 1999; Pb. CET 2000]
Question#10
Which of the following does not give brick red precipitate with Fehling solution [AIIMS 1996]
Question#11
Acetaldehyde and acetone differ in their reaction with [KCET 1989]
Question#12
Fehling's test is positive for [KCET 1993]
Question#13
Which of the following does not turn Schiff's reagent to pink [DPMT 1981; CPMT 1989]
Question#14
CH3CH =CHCHO is oxidised to CH3CH= CHCOOH using [NCERT 1978]
Question#15
Silver mirror is a test for [DPMT 1983; CBSE PMT 1988]
Question#16
Aldehydes can be oxidised by [NCERT 1983]
Question#17
The alkaline CuSO4 containing sodium potassium tartrate does not react with [MP PMT 1997]
Question#18
The light yellow compound produced when acetone reacts with iodine and alkali, is [MP PMT 1992; EAMCET 1993]
Question#19
Dimethyl ketones are usually characterised through [MNR 1992]
Question#20
Haloform test is given by the following substance [EAMCET 1988]
Question#21
Which of the following will not give the iodoform test [MNR 1994]
Question#22
Acetaldehyde cannot show [AIIMS 1997]
Question#23
C2H5CHO and (CH 3 )2CO can be distinguished by testing with [EAMCET 1998; CPMT 1994, 97; MP PET 1995; MP PMT 1996; RPMT 1997, 99]
Question#24
Which of the following organic compounds exhibits positive Fehling test as well as iodoform test [MP PET 1994; KCET 2001]
Question#25
Which of the following is incorrect [CBSE PMT 2001]
Question#26
Methyl ketones can be characterized by performing NUMS2018
Question#27
Which one the following compound will produced a yellow precipitate with I2 dissolve in NaOH SET2019
Question#28
Which one the following compound will produced a yellow precipitate with I2 dissolve in NaOH SET2019
Question#29
Which one of the following reagent can be used to detect an aldehyde ? SET2019
Question#30
Which of the following will give a positive test with Tollens reagent MDCAT2019
Question#31
Identification test for functional group of organic compound are associated with specific observation. Tollen's reagent is ammonical silver nitrate solution which is used for identification of a functional group ''X'' with an observation ''O'' . Identify X and O MDCAT2019
Question#32
\( CH_3-CH_2-OH \xrightarrow[H2SO4]{K2Cr2O7} \ M \ \xrightarrow{AgNO3 + NH4OH} N \) MDCAT2019
What is the final indication in given reaction
Question#33
Which of the following will give iodoform test on treatment with aqueous iodine ? MDCAT2017
Question#34
To distinguish aldehyde from ketone which solution is used ? MDCAT2017
Question#35
Which of the following test is given by both aldehyde and ketone ? MDCAT2016
Question#36
Both aldehyde and ketone give ___________ ? MDCAT2017
Question#37
Which one of the following is also called silver mirror test MDCAT2015
Question#38
Which group gives a yellow precipitate of triiodo-methane when warmed with alkaline aqueous iodine? MDCAT2013
Question#39
Consider the following reaction: MDCAT2012
\( R-CHO + 2[Ag[NH_3)_2]OH \rightarrow RCOONH_4 + 2Ag + 2NH_3 + H_2O \)
this reaction represents which of the following test
Question#40
Brick red precipitate are formed when aldehyde reacts with MDCAT2010
Question#41
Which of following will give a positive test with tollens reagent? MDCAT2019
Question#42
Which of the following compound will give a secondary alcohol after reaction with NaBH4 MDCAT2019
Question#43
Which of the following reagent is use to separate and purify carbonyls and non-carbonyl compounds MDCAT2020