Home/Chemistry Of Hydrocarbons
Reactivity Of alkene
Question#1
Addition reaction of 2-butene with HBr produces. [MDCAT2023]
Question#2
Which reactions are most common in alkenes [Pb. CET 1989]
Question#3
Which reactions are most common in alkenes [Pb. CET 1989]
Question#4
Markownikoff's rule provides guidance of addition of HBr on [MNR 1994]
Question#5
Markownikoff's rule provides guidance of addition of HBr on [MNR 1994]
Question#6
Ethene gives with acidic KMnO4 solution [MP PMT 1997]
Question#7
Which of the following is the most stable alkene [AIIMS 1998; KCET (Med.) 2000; CPMT 2003]
Question#8
Cyclopentene on treatment with alkaline KMnO4 gives [CPMT 1987]
Question#9
Cyclopentene on treatment with alkaline KMnO4 gives [CPMT 1987]
Question#10
In a reaction, if half of the double bond is broken and two new bonds are formed, this is a case of [AMU 1983; NCERT 1978; CPMT 1983]
Question#11
Oils are converted into fats by [Kerala (Med.) 2002]
Question#12
Oils are converted into fats by [Kerala (Med.) 2002]
Question#13
1, 3-butadiene reacts with ethylene to form [BHU 2001]
Question#14
A hydrocarbon X adds on one mole of hydrogen to give another hydrocarbon and de-colourized bromine water. X reacts with KMnO4 in presence of acid to give two moles of the same carboxylic acid. The structure of X is [JIPMER 2001]
Question#15
Which of the following is not used to distinguish ethene from ethane [KCET (Med.) 2001; UPSEAT 2002; CBSE PMT 2002]
Question#16
One mole of each of the following alkenes is catalytically hydrogenated. The quantity of heat evolved will be the lowest in the case of [Roorkee 2000]
Question#17
Which of the following compound is produced when \( CH_2 =CH - (CH_2 )_2-COOH \) reacts with HBr in presence of peroxides [AIIMS 2000]
Question#18
Isopropyl alcohol is obtained by reacting which of the following alkenes with conc. H2 SO4 and H2O [MP PMT 1999]
Question#19
Baeyer's reagent is used in the laboratory for [CBSE PMT 1991, 92; AIIMS 1998; AFMC 1999]
Question#20
Aqueous sulphuric acid reacts with 2-methyl-1-butene to give predominantly [Roorkee 1992]
Question#21
Dilute aqueous KMnO4 , at room temperature reacts with \( R -CH = CH - R \) to give [Roorkee 1992]
Question#22
Which one of the following characteristics apply to both ethene and ethyne [NCERT 1990]
Question#23
In which of the following, addition of HBr does not take place against Markownikoff's rule or Anti-Markownikoff addition of HBr is not observed for [IIT-JEE 1985; CBSE PMT 1994; MADT Bihar 1995; MP PMT 1999; AMU 2002]
Question#24
Ozonolysis of which one of the following will give two molecules of acetaldehyde [Bihar MEE 1997; MP PET 2000]
Question#25
When 3, 3-dimethyl-2-butanol is heated with H2 SO4 the major product obtained is [CBSE PMT 1995]
Question#26
The disappearance of the characteristic purple colour of KMnO4 in its reaction with an alkene is the test for unsaturation. It is known as [CPMT 1989, 94; CBSE PMT 1990]
Question#27
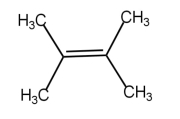 \( \xrightarrow[KOH/heat]{X} \)
\( \xrightarrow[KOH/heat]{X} \) 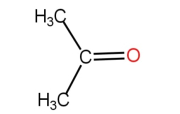 +
+ 2.jpeg) X
in the above reaction is [CPMT 1985, 93]
X
in the above reaction is [CPMT 1985, 93]
Question#28
The propene reacts with HBr to form [AIIMS 1999; RPET 1999]
Question#29
Alkenes usually show which type of reaction [AIIMS 1999; MADT Bihar 1980]
Question#30
Ethene when treated with Br2 in the presence of CCl 4 which compound is formed [RPMT 1997; DCE 2001; KCET (Med.) 1999]
Question#31
Addition of bromine to 1, 3-butadiene gives [CPMT 1987, 93]
Question#32
Propene reacts with hypochlorous acid to form ETEA2017
Question#33
Choose the least stable of the following butene ETEA2017
Question#34
Alkenes undergo MDCAT2019
Question#35
Which of the following reactions is used for the production of alcohols on industrial scale MDCAT2019
Question#36
In the reaction sequence: MDCAT2019
\( H_3C-CH_2-CH_2-Br \xrightarrow{\text{AlcKOH}} C \xrightarrow[\text{H2O}]{\text{H2SO4}} D \)
Product D will be
Question#37
Which of the options show all possible products of combustion of butene SET2019
Question#38
Bromination of alkene is shown in the following reaction. the reaction is used for MDCAT2018
\( CH_2=CH_2 + Br_2 \rightarrow CH_3-CH_3 \)
Question#39
Chlorination and Bromination mostly uses MDCAT2017
Question#40
Reactivity order of alkene with hydrogen halide is MDCAT2015
Question#41
Addition of unsymmetrical reagent to unsymmetrical alkene is governed by MDCAT2014
Question#42
Hydrogenation of unsaturated oils is done by using MDCAT2011
Question#43
Ethene on polymerization, give the product polyethene, this reaction may be called as MDCAT2012
Question#44
What is the product formed when propene reacts with HBr. MDCAT2013
Question#45
Compound X on reaction with O3 followed by Zn/H2O gives formaldehyde and 2-methyl propanal NEET2022