Home/Electrochemistry
Standard hydrogen electrode
Question#1
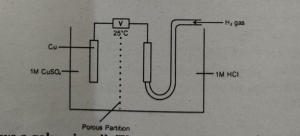
The diagram show a galvanic cell, the current flows from MDCAT2016
Please select an option
Solution:
Question#2
Coinage metals Cu, Ag and Au are the least reactive because they have MDCAT2016
Please select an option
Solution:
Question#3
The standard electrode potential of hydrogen is arbitrarily taken at 298k is ______ MDCAT2018
Please select an option
Solution:
Question#4
The Eo value of standard copper half-cell is 0.34V, measured when it is connected with SHE i.e standard hydrogen electrode. In this case the half reaction taking place at SHE is MDCAT2017
Please select an option
Solution:
Related MCQ's Catrgories
Categories
1. Introduction To Fundamental
2. Atomic Strcuture
6. Chemical Equilibrium
7. Reaction kinetics
8. Thermochemistry
9. Electrochemistry
11. s and p block Elements
14. Chemistry Of Hydrocarbons
15. Benzene
16. Alcohol and phenols
18. Aldehyde and ketones
19. Carboxylic acid
20. Macromolecules