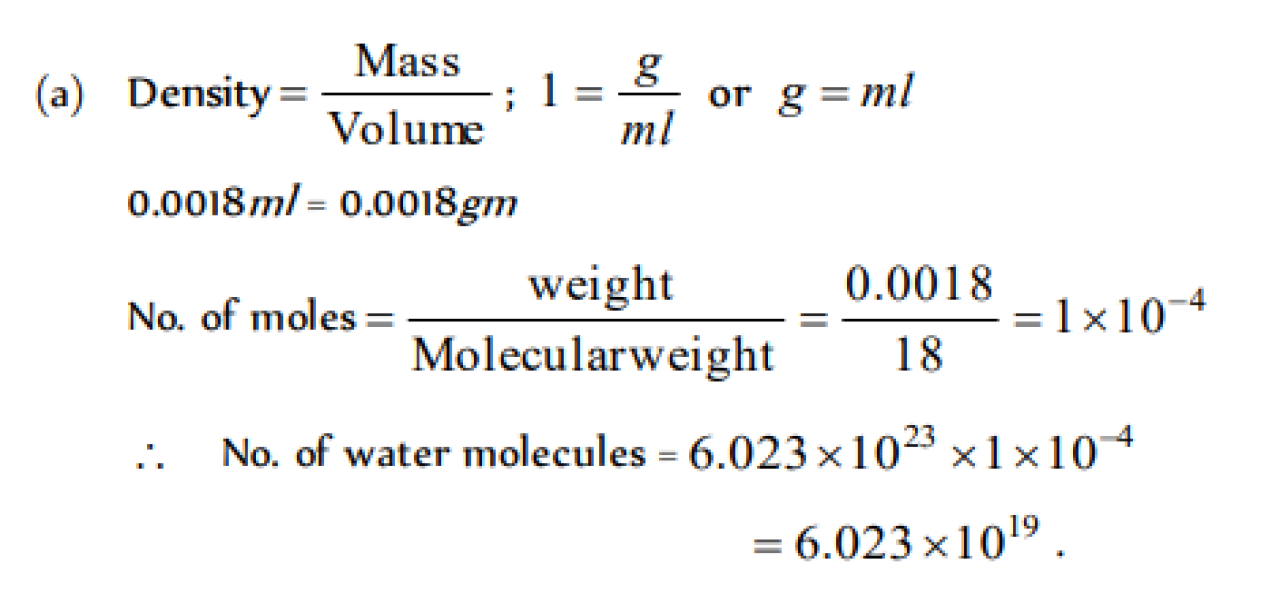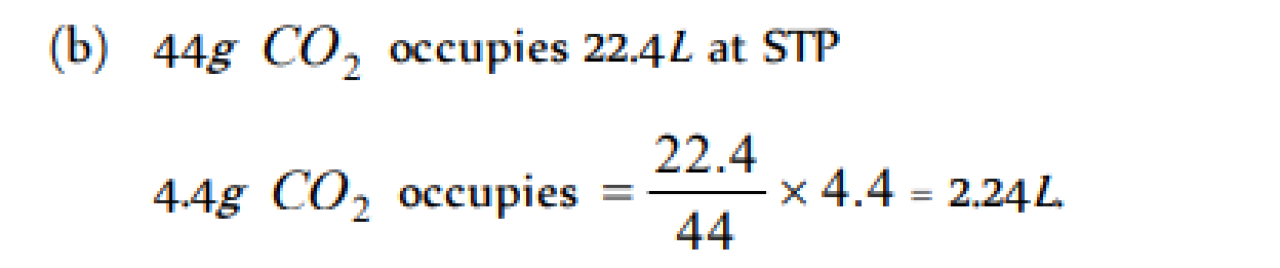Home/Introduction To Fundamental
Concept of Mole
Question#1
Number of moles in an element is directly proportional to (MDCAT-2024)
Question#2
10g of H2 has same number of molecules as in (NEET-2024)
Question#3
The same mole of N2 , H2 and O2 are present in one 0.1cc of volume at STP, which one has greater number of molecules MDCAT2023
Question#4
In a mole of water vapour at STP, the volume actually occupied or taken by the molecules (i.e., Avogadro’s No. x Volume of one molecule) is [Kerala EEE 2000]
Question#5
The number of molecules of CO2 present in 44g of CO2 is [BCECE 2005]
Question#6
How many atoms are contained in one mole of sucrose C12H22O11 [Pb. PMT 2002]
Question#7
The number of moles of sodium oxide in 620g of it is [BHU 1992]
Question#8
The largest number of molecules is in [BHU 1997]
Question#9
How many molecules are present in one gram of hydrogen [AIIMS 1982]
Question#10
The number of electrons in a mole of hydrogen molecule is [CPMT 1987]
Question#11
The numbers of moles of BaCO3 which contain 1.5 moles of oxygen atoms is [EAMCET 1991]
Question#12
The number of water molecules in 1 L of water is [EAMCET 1990]
Question#13
Number of molecules in 100 ml of each of O2 , NH3 and CO2 at STP are [Bihar MADT 1985]
Question#14
19.7 kg of gold was recovered from a smuggler. How many atoms of gold were recovered (Au =197) [Pb. CET 1985]
Question#15
One mole of calcium phosphide on reaction with excess of water gives [IIT 1999]
Question#16
The number of water molecules present in a drop of water (volume 0.0018 ml) at room temperature is [DCE 2000]
Question#17
The volume occupied by 4.4 g of CO2 at STP is [AFMC 1997, 2004; Pb. CET 1997, 2002]
Question#18
The number of oxygen atoms in 4.4 g of CO2 is approx. [CBSE PMT 1990]
Question#19
A molar solution is one that contains one mole of a solute in [IIT 1986]
Question#20
Which one of the following pairs of gases contains the same number of molecules [EAMCET 1987]
Question#21
The number of molecules in 4.25 g of ammonia are [Pb. CET 2000]
Question#22
The number of moles of oxygen in 1 L of air containing 21% oxygen by volume, in standard conditions, is [CBSE PMT 1995; Pb. PMT 2004]
Question#23
4.4 g of an unknown gas occupies 2.24L of volume at standard temperature and pressure. The gas may be [MP PMT 1995]
Question#24
How many mole of helium gas occupy 22.4 L at 0oC at 1 atm. pressure [Kurukshetra CEE 1992; CET 1992]
Question#25
How many mole of CO2 contain 16g of oxygen ? NUMS2021
Question#26
Question#27
How many atoms are contained in one mole of Ca(OH)2 ETEA2010 Medical QNo.115
Question#28
The number of atoms in 4.25 g of NH3 is approximately [CBSE PMT 1999; MH CET 2003]
Question#29
The number of electrons in a mole of hydrogen molecule is [CPMT 1987]
Question#30
Which of following has maximum number of molecule among the following 2011Mains
Question#31
The number of atoms in 0.1 mol of gas a tri atomic gas is NEET2010
Question#32
Which of following has maximum number of atoms NEET2020
Question#33
The number of gram-atom in 3g of hydrogen atom is the same as the number of gram atom in 48g of ETEA2018
Question#34
A metal has total surface area of 150cm3i. It is evenly coated with silver by electrolysis. Its mass increases by 0.216g.
How many atoms of Silver are deposited per cm3 on the surface of the metal? Cambridge International AS & A level-2022
Question#35
Nauman has body mass 60.0kg. He has 18% water in his body. Total number of water molecules in his body are MDCAT-2017
Question#36
Number of moles of O2 in 10.6g of Na2CO3 MDCAT2017
Question#37
Choose the correct option regarding number of particles associated with 1 mole of a substance MDCAT2017
Question#38
In which case number of molecules of water are maximum? NEET-2018
Question#39
3.0mole of calcium will contained ____________g of calcium MDCAT2018
Question#40
What mass of 95% pure CaCO3 will be required to neutralize 50ml of 0.5M HCl solution according to following reaction.
CaCO3 + 2HCl----------> CaCl2 + CO2 + 2H20 NEET-2022
Question#41
Number of mole of water in 1kg of Ice are [MDCAT2019]
Question#42
What Mass of NaOH is present in 0.5mole of Sodium Hydroxide MDCAT2016
Question#43
In a vessel 10g of N2 ,10g H2, 10g O2 are present. which one will have least no of atoms? [MdCAT2020]