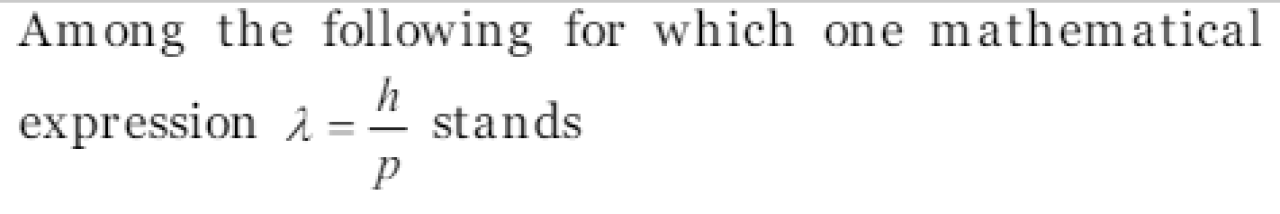Home/Atomic Strcuture
de Broglie relation
Question#1
Which is the correct relationship between wavelength and momentum of particles [Pb. PMT 2000]
Question#2
The de-Broglie equation applies [MP PMT 2004]
Question#3
Calculate the de-Broglie wavelength of an electron traveling at 1% of the speed of light [DPMT 2004]
Question#4
Dual nature of particles was proposed by [DCE 2004]
Question#5
A cricket ball of 0.5 kg is moving with a velocity of 100m /sec . The wavelength associated with its motion is [DCE 2004]
Question#6
A 200g golf ball is moving with a speed of 5 m per hour. The associated wavelength is ( h = 6.625 x 10-34 J - sec [MP PET 2003]
Question#7
If the velocity of hydrogen molecule is 5 x 104 cm sec-1 , then its de-Broglie wavelength is [MP PMT 2003]
Question#8
What is the de-Broglie wavelength associated with the hydrogen electron in its third orbit [A MU (En gg.) 2002]
Question#9
The de-Broglie wavelength associated with a particle of mass 10-6 kg moving with a velocity of 10 ms-1 , is [A IIMS 2001]
Question#10
What will be the de-Broglie wavelength of an electron moving with a velocity of 1.2 x 105 ms-1 [MP PET 2000]
Question#11
An electron has kinetic energy 2.8 x 10-23 J . de-Broglie wavelength will be nearly . ( me kg 9.1 x 10-31 kg ) [MP PET 2000]
Question#12
The de-Broglie wavelength associated with a material particle is [JIPMER 2000]
Question#13
Minimum de-Broglie wavelength is associated with [RPMT 1999]
Question#14
The de-Broglie wavelength of a particle with mass 1gm and velocity 100m / sec is [CBSE PMT 1999; EA MCET 1997; A FMC 1999; AIIMS 2000]
Question#15
de-Broglie equation is [MP PMT 1999; CET Pune 1998]
Question#16
Which of the following expressions gives the de-Broglie relationship [MP PMT 1996, 2004; MP PET /PMT 1998]
Question#17
Which one of the following explains light both as a stream of particles and as wave motion [A IIMS 1983; IIT 1992; UPSEAT 2003]
Question#18
Question#19
The wave nature of an electron was first given by [CMC V Vellore 1991; Pb. PMT 1998; CPMT 2004]
Question#20
De Broglie equation describes the relationship of wavelength associated with the motion of an electron and its [MP PMT 1986]