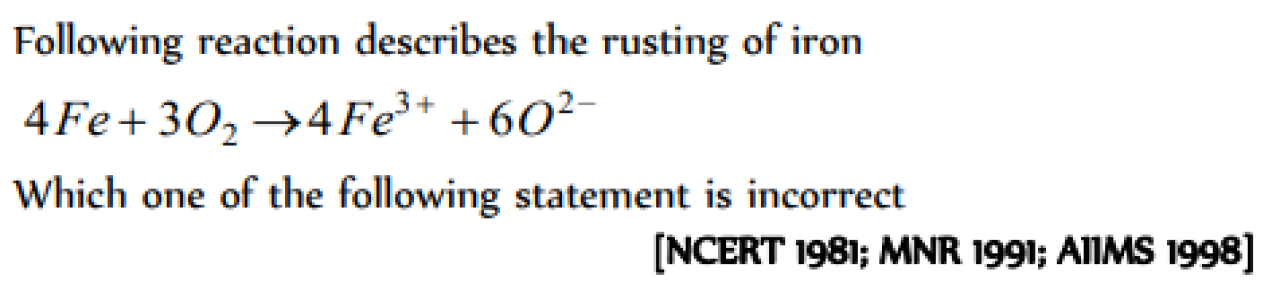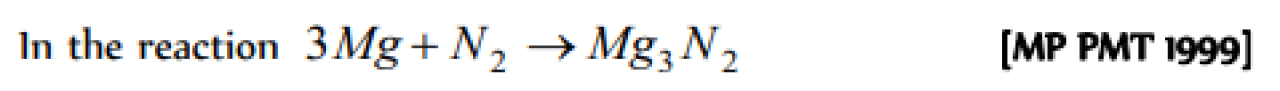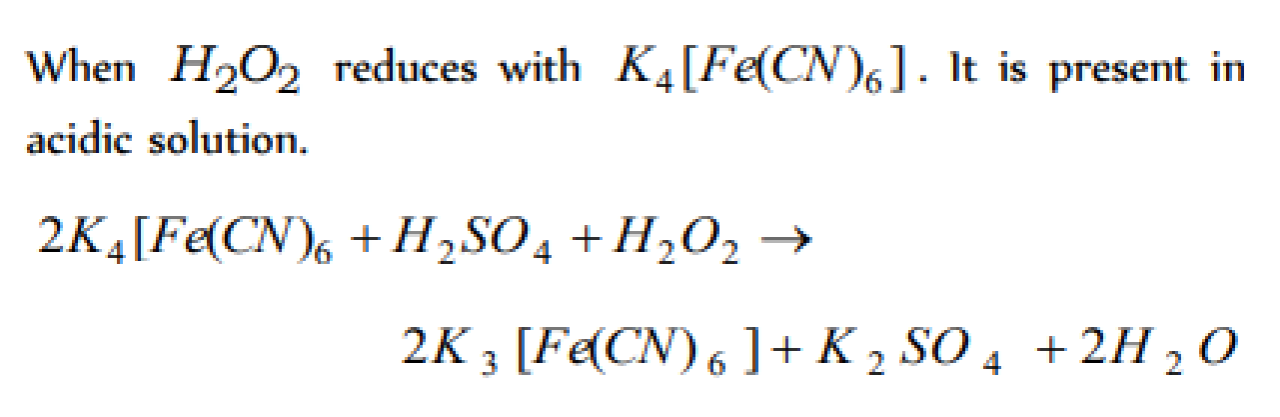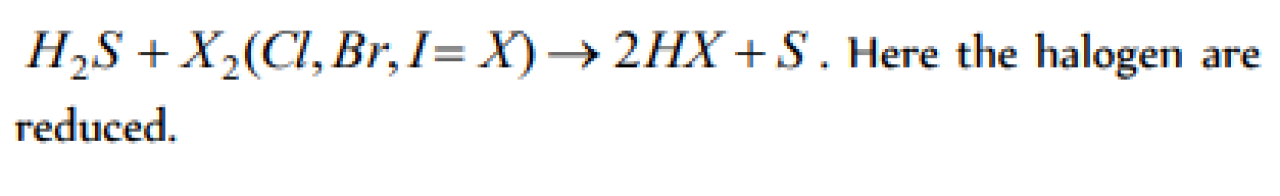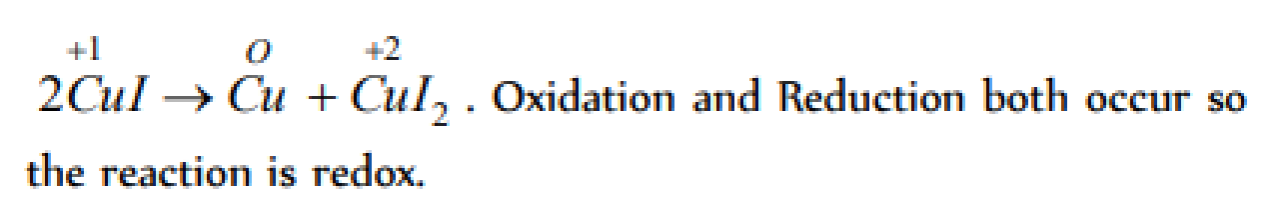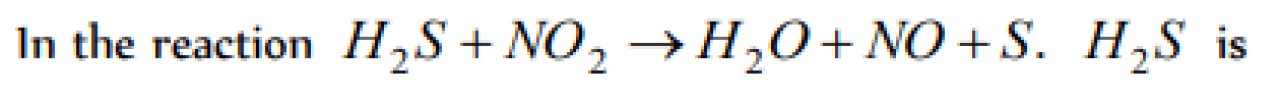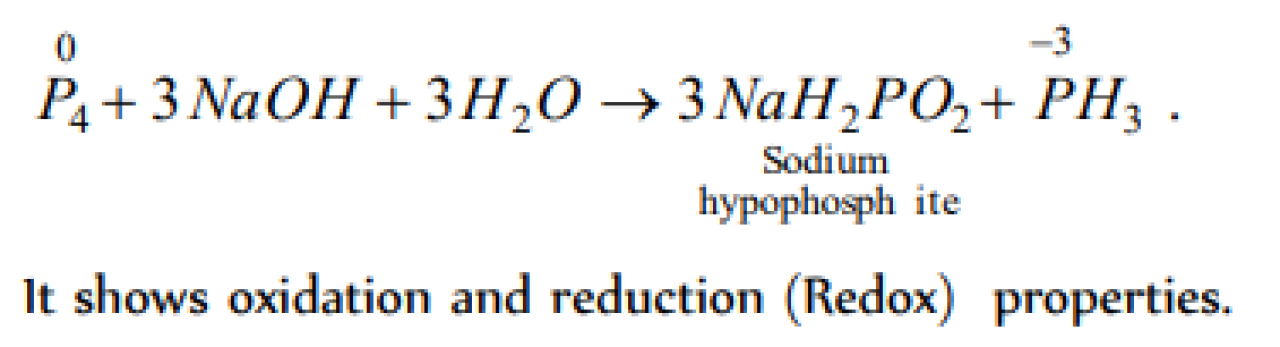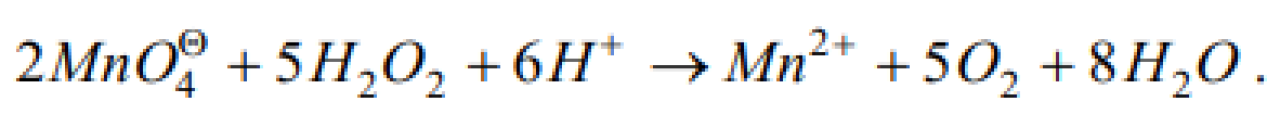Home/Electrochemistry
Oxidation and reduction
Question#1
Which of the following is redox reaction [CBSE PMT 1997]
Question#2
Question#3
When iron or zinc is added to CuSO4 solution, copper is precipitated. It is due to [CPMT 1974, 79]
Question#4
Question#5
When Fe2+ changes to Fe3+ in a reaction
Question#6
Which halide is not oxidised by MnO2 [MNR 1985; JIPMER 2000]
Question#7
Question#8
Question#9
Question#10
Question#11
Which one of the following reactions does not involve either oxidation or reduction [EAMCET 1982]
Question#12
In a reaction between zinc and iodine, in which zinc iodide is formed, what is being oxidised [NCERT 1975]
Question#13
Reduction involves [ NCERT 1972]
Question#14
One gas bleaches the colour of flowers by reduction while the other by oxidation [EAMCET 1980]
Question#15
Question#16
Oxidation of thiosulphate ( S2O32- ) ion by iodine gives [NCERT 1976]
Question#17
When Sn2+ changes to Sn4+ in a reaction [CPMT 1981]
Question#18
Solution of sodium metal in liquid ammonia is strongly reducing due to the presence of the following in the solution [NCERT 1977; KCET (Med.) 2000]
Question#19
When copper turnings are added to silver nitrate solution, a blue-coloured solution is formed after some time. It is because, copper [CPMT 1974, 79; DPMT 2000]
Question#20
Incorrect statement regarding rusting is [MP PET 2000]
Question#21
Oxidation involves [NCERT 1971, 81; CPMT 1980, 82, 83; MP PMT 1983]
Question#22
SnCl2 gives a precipitate with a solution of . HgCl2 In this process HgCl2 is [CPMT 1983]
Question#23
Question#24
When sodium metal is dissolved in liquid ammonia, blue colour solution is formed. The blue colour is due to [NCERT 1981]
Question#25
Question#26
Max. number of moles of electrons taken up by one mole of NO3_ when it is reduced to [DPMT 2002]
Question#27
H2O2 reduces K4Fe(CN)6 [MP PMT 1985]
Question#28
H2S reacts with halogens, the halogens [JIPMER 2000]
Question#29
Question#30
In the course of a chemical reaction an oxidant [MP PMT 1986]
Question#31
The conversion of PbO2 to Pb(NO3)2 is
Question#32
Question#33
Which one of the following does not get oxidised by bromine water [MP PET/PM 2001]
Question#34
When P reacts with caustic soda, the products are PH3 and . NaH2PO2 This reaction is an example of [IIT 1980; Kurukshetra CEE 1993; CPMT 1997]
Question#35
The ultimate products of oxidation of most of hydrogen and carbon in food stuffs are [DCE 2001]
Question#36
When a sulphur atom becomes a sulphide ion [AMU 1999]
Question#37
H2O2 reduces MnO4- ion to [KCET (Med.) 2000]
Question#38
The oxidation number of carbon in Mg(HCO3)2? ; (2018-ETEA)
Question#39
Oxidation number of an element in free state is NUMS2021
Question#40
Reductions occurs at NUMS2021
Question#41
In which of following, oxygen shows fractional oxidation number MDCAT-2022
Question#42
The common oxidation number of halogens is MDCAT2020
Question#43
Question#44
The oxidation state of nitrogen in NH4NO3 are ETEA2019
Question#45
Rusting of Iron metal occurs when Fe gets converted into Fe2O3. What happened with Fe? SET2019
Question#46
In all oxidation reaction, atoms of an element in a chemical specie lose electrons and increases their MDCAT2011 MDCAT2020
Question#47
In MgCl2 the oxidation state of Cl is MDCAT2012
Question#48
In SO4-2 the oxidation number of Sulphur is MDCAT2016
Question#49
The value of oxidation number of Cl in HClO3 is SET2019
Question#50
The oxidation state of carbon in C2O4-2 is NUMS2019
Question#51
In NO3-1 the oxidation number of N is MDCAT2017