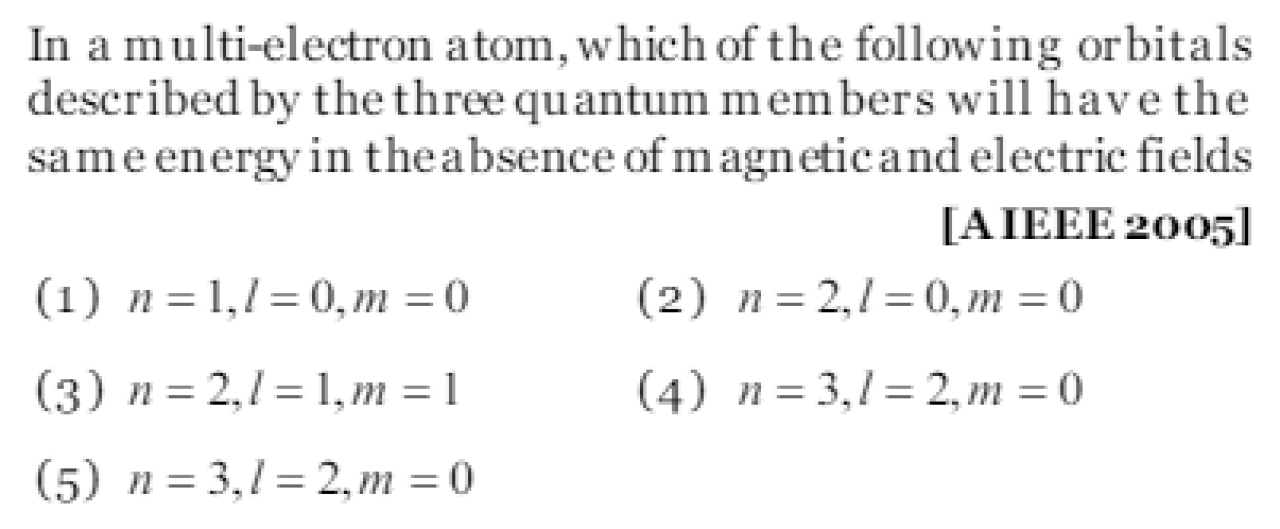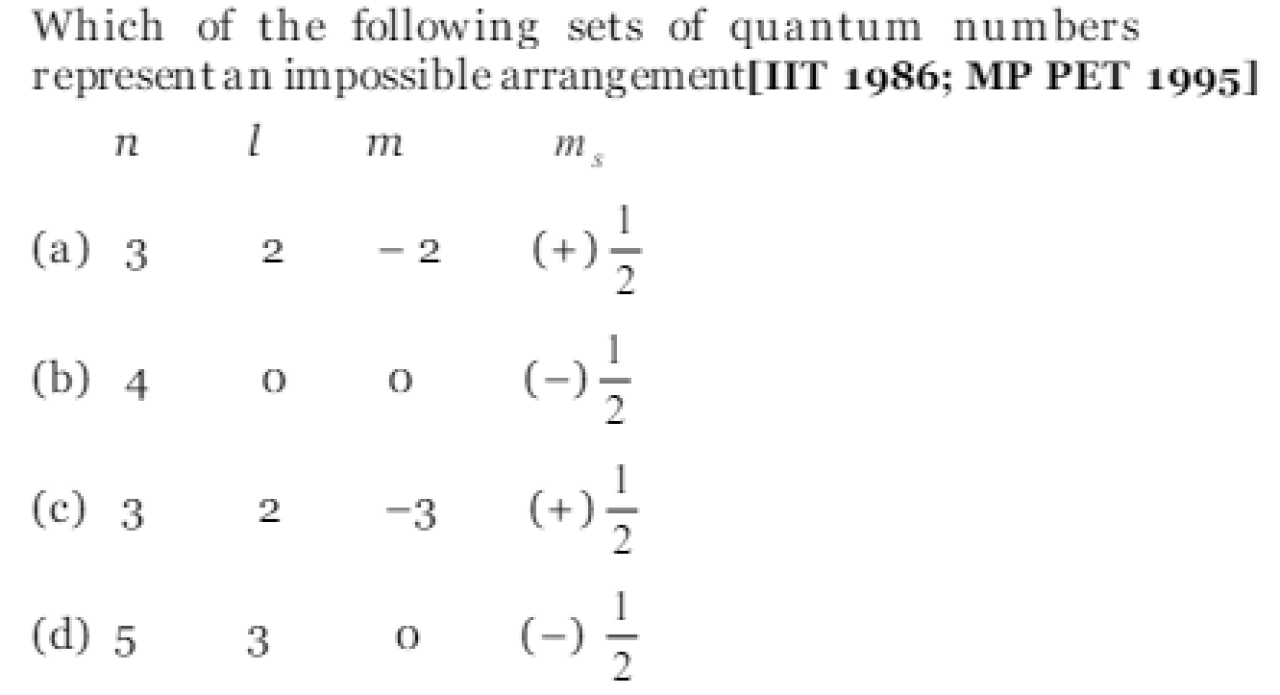Question#1
The p orbital has MDCAT2024
Question#2
The quantum number of four electrons are given below:
- I) n=4 ; l=2 m=-2 s= -1/2
- II) n=3 ; l =2 m=1 s= +1/2
- III) n=4 ; l=1 m=0 s=+1/2
- IV) n=3 ; l=1 m=-1 s=+1/2
Correct decreasing order of energy of these electrons is (NEET-2024)
Question#3
A 3p orbital has [IIT 1995]
Question#4
Azimuthal quantum number for last electron of Na atom is [BHU 1995]
Question#5
Correct configuration of Fe+3 [26] is [CPMT 1994; BHU 1995; KCET 1992]
Question#6
Which of the following statements is not correct for an electron that has the quantum numbers n = 4 and m = 2 [MNR 1993]
Question#7
Which of the following represents the correct sets of the four quantum numbers of a 4d electron [MNR 1992; UPSEAT 2001; J&K CET 2005]
Question#8
The quantum number which may be designated by s, p, d, and f instead of number is BHU 1980]
Question#9
An ion has 18 electrons in the outermost shell, it is [CBSE PMT 1990]
Question#10
For azimuthal quantum number l = 3, the maximum number of electrons will be [CBSE PMT 1991; EA MCET 1991; RPMT 2002; CBSE PMT 2002]
Question#11
How many electrons can be accommodated in a sub-shell for which n = 3,l = 1 [CBSE PMT 1990]
Question#12
When the azimuthal quantum number has a value of l = 1, the shape of the orbital is [MP PET 1993]
Question#13
The principal quantum number represents [CPMT 1991]
Question#14
The electronic configuration (outermost) of Mn+2 ion (atomic number of ( Mn = 25 ) in its ground state is [MP PET 1993]
Question#15
The shape of p -orbital is [MP PMT 1993]
Question#16
The electronic configuration of chromium is [MP PMT 1993; MP PET 1995; BHU 2001; BCECE 2005]
Question#17
There is no difference between a 2p and a 3p orbital regarding [BHU 1981]
Question#18
Electronic configuration of C is [CPMT 1975]
Question#19
The total number of electrons that can be accommodated in all the orbitals having principal quantum number 2 and azimuthal quantum number 1 is [CPMT 1971, 89, 91]
Question#20
The azimuthal quantum number is related to [BHU 1987, 95]
Question#21
If an electron has spin quantum number of + 1/2 and a magnetic quantum number of -1 , it cannot be presented in an [CBSE PMT 1989; UPSEA T 2001]
Question#22
If an electron has spin quantum number of + 1/2 and a magnetic quantum number of -1 , it cannot be presented in an [CBSE PMT 1989; UPSEA T 2001]
Question#23
If the value of azimuthal quantum number is 3, the possible values of magnetic quantum number would be [MP PMT 1987; RPMT 1999; AFMC 2002; KCET 2002]
Question#24
How many electrons can be fit into the orbitals that comprise the 3rd quantum shell n = 3 [MP PMT 1986, 87; Orissa JEE 1997]
Question#25
In a potassium atom, electronic energy levels are in the following order [EA MCET 1979; DPMT 1991]
Question#26
When 3d orbital is complete, the new electron will enter the [EA MCET 1980; MP PMT 1995]
Question#27
The shape of s -orbital is [NCERT 1978I]
Question#28
Question#29
The magnetic quantum number for valency electrons of sodium is [CPMT 1988; MH CET 1999]
Question#30
When the azimuthal quantum number has a value of l = 0, the shape of the orbital is [MP PET 1995]
Question#31
For n = 3 energy level, the number of possible orbitals (all kinds) are [BHU 1981; CPMT 1985; MP PMT 1995]
Question#32
For d electrons, the azimuthal quantum number is [MNR 1983; CPMT 1984]
Question#33
Pauli's exclusion principle states that [MNR 1983; A MU 1984]
Question#34
A sub-shell l = 2 can take how many electrons [NCERT 1973, 78]
Question#35
The number of orbitals in d sub-shell is [MNR 1981]
Question#36
The number of orbitals in 2p sub-shell is [NCERT 1973; MP PMT 1996]
Question#37
The four quantum numbers of the outermost orbital of K (atomic no. =19) are [MP PET 1993, 94]
Question#38
The number of radial nodes of 3 s and 2p orbitals are respectively. [IIT-JEE 2005]
Question#39
The explanation for the presence of three unpaired electrons in the nitrogen atom can be given by [NCERT 1979; RPMT 1999; DCE 1999, 2002; CPMT 2001; MP PMT 2002; Pb. PMT / CET 2002]
Question#40
The four quantum number for the valence shell electron or last electron of sodium (Z = 11) is [MP PMT 1999]
Question#41
The total number of orbitals in an energy level designated by principal quantum number n is equal to [A IIMS 1997; J&K CET 2005]
Question#42
If magnetic quantum number of a given atom represented by –3, then what will be its principal quantum number [BHU 2005]
Question#43
A completely filled d-orbital ( d10 ) [MNR 1987]
Question#44
The two electrons in K sub-shell will differ in [MNR 1988; UPSEAT 1999, 2000; Kerala PMT 2003]
Question#45
The structure of external most shell of inert gases is [JIPMER 1991]
Question#46
For the dumb-bell shaped orbital, the value of l is [CPMT 1987, 2003]
Question#47
The valence electron in the carbon atom are [MNR 1982]
Question#48
The number of electrons in the valence shell of calcium is [IIT 1975]
Question#49
The following has zero valency [DPMT 1991]
Question#50
The magnetic quantum number for an electron when the value of the principal quantum number is 2 can have [CPMT 1984]
Question#51
The shape of 2p orbital is [CPMT 1983; NCERT 1979]
Question#52
. The total number of unpaired electrons in d-orbitals of atoms of element of atomic number 29 is [CPMT 1983]
Question#53
Which orbital is dumb-bell shaped [MP PMT 1986; MP PET/PMT 1998]
Question#54
If n = 3, then the value of 'l' which is incorrect [CPMT 1994]
Question#55
Question#56
The magnetic quantum number specifies [MNR 1986; BHU 1982; CPMT 1989, 94; MP PET 1999; AFMC 1999; AMU (Engg.) 1999]
Question#57
Principal quantum number of an atom represents [EA MCET 1979; IIT 1983; MNR 1990;UPSEAT 2000, 02]
Question#58
The quantum numbers for the outermost electron of an element are given below as n = 2, l = 0, m = 0,s = +1/2. The atoms is [EA MCET 1978]
Question#59
2p orbitals have [NCERT 1981; MP PMT 1993, 97]
Question#60
Correct set of four quantum numbers for valence electron of rubidium (Z = 37 ) is [IIT 1984; JIPMER 1999; UPSEAT 2003]
Question#61
Principal, azimuthal and magnetic quantum numbers are respectively related to [CPMT 1988; A IIMS 1999]
Question#62
The shape of an orbital is given by the quantum number [NCERT 1984; MP PMT 1996]
Question#63
The quantum number which specifies the location of an electron as well as energy is [DPMT 1983]
Question#64
Which quantum number is not related with Schrodinger equation [RPMT 2002]
Question#65
The maximum probability of finding an electron in the dxy orbital is [MP PET 1996]
Question#66
Who modified Bohr's theory by introducing elliptical orbits for electron path [CBSE PMT 1999; A FMC 2003]
Question#67
The number of electrons in the p-subshell is NUMS2021
Question#68
Which quantum number tells shape of the orbitals NUMS2021
Question#69
p-orbitals of an atom in presence of magnetic field are [Pb. PMT 2002]
Question#70
Which of following element in its atomic state has electrons fully occupying first two spherically symmetrical orbitals [SET-2019]
Question#71
Which of the following sets of quantum numbers is correct for an electron in 4 f orbital [A IEEE 2004]
Question#72
For principle quantum number n =4 the total number of orbitals having l = 3 [A IIMS 2004]
Question#73
Quantum numbers of an atom can be defined on the basis of [AIIMS 2002]
Question#74
An e- has magnetic quantum number as -3 , what is its principal quantum number [BHU 1998]
Question#75
For azimuthal quantum number l =3 , the maximum number of electrons will be [CBSE PMT 1991; EA MCET 1991; RPMT 2002; CBSE PMT 2002]
Question#76
The total number of electrons that can be accommodated in all the orbitals having principal quantum number 2 and azimuthal quantum number 1 is [CPMT 1971, 89, 91]
Question#77
With increase in the value of principle quantum number ''n'' the shape of the ''s'' orbitals remain same although their sizes MDCAT2012
Question#78
The four quantum number for the valence shell electron or last electron of sodium (Z = 11) is [MP PMT 1999]
Question#79
The total number of orbitals in an energy level designated by principal quantum number n is equal to [AIIMS 1997; J&K CET 2005]
Question#80
A completely filled d -orbital ( d10 )[MNR 1987]
Question#81
The two electrons in K sub-shell will differ in [MNR 1988; UPSEAT 1999, 2000; Kerala PMT 2003]
Question#82
The shape of 2p orbital is [CPMT 1983; NCERT 1979]
Question#83
An orbital can never occupied by ETEA 2009-58 Medical
Question#84
How many different values can m have in the subshell designed by quantum number n=5 l=4 ETEA-2013-108-Medical
Question#85
choose the atom that is not having a spin quantum number 1/2 ETEA2016-87medical
Question#86
The total number of unpaired electrons in d - orbitals of atoms of element of atomic number 29 is [CPMT 1983]
Question#87
Which orbital is dumb-bell shaped [MP PMT 1986; MP PET/PMT 1998]
Question#88
If n =3 , then the value of 'l' which is incorrect [CPMT 1994]
Question#89
The magnetic quantum number specifies [MNR 1986; BHU 1982; CPMT 1989, 94; MP PET 1999; AFMC 1999; AMU (Engg.) 1999]
Question#90
Principal quantum number of an atom represents [EA MCET 1979; IIT 1983; MNR 1990;UPSEAT 2000, 02]
Question#91
Principal, azimuthal and magnetic quantum numbers are respectively related to [CPMT 1988; A IIMS 1999]
Question#92
The quantum number which specifies the location of an electron as well as energy is [DPMT 1983]
Question#93
Match List I with list II NEET-2022
| a) n=2 l=1 | i) 2s |
b) n=3 l=2 | ii) 3s |
| c) n=3 l=0 | iii) 2p |
| d) n=2 l=0 | iv) 3d |
Question#94
Maximum number of electrons in a sub shell is given by NUMS2019
Question#95
What are values of principle quantum number and azimuthal quantum number for last electron in chlorine atom. ETEA2016
Question#96
Quantum number which describe the orientation of orbitals in three dimension space is ETEA-2019
Question#97
Which of the following set of quantum number is possible AIIMS-2001
Question#98
Identify the incorrect statement from the following NEET-2022
Question#99
The relationship between quantum number n and l is MDCAT2020