Question#1
What is the IUPAC name of diisopropyl ketone. [MDCAT2023]
Question#2
Formaldehyde reacts with ammonia to give urotropine. The formula of urotropine is [MP PMT 1989, 96, 2003; AIIMS 1982; NCERT 1987; MP PET 1990, 91, 2000; CPMT 1978, 82, 86, 97; KCET 2003]
Question#3
Acetone and acetaldehyde are [KCET 1998]
Question#4
Which of the following is a mixed ketone [AFMC 1997]
Question#5
IUPAC name of CCl3CHO is [MP PMT/PET 1988]
Question#6
What is the compound called if remaining two valencies of a carbonyl group are satisfied by two alkyl groups [CPMT 1990]
Question#7
What is the compound called if remaining two valencies of a carbonyl group are satisfied by two alkyl groups [CPMT 1990]
Question#8
IUPAC name of CH3COCH3 is [MP PET 1991]
Question#9
Glyoxal is [BVP 2003]
Question#10
The IUPAC name of the following structure is [MP PMT 1995]
.jpeg)
Question#11
Which of the following compounds does not contain an -OH group [CPMT 1982]
Question#12
In aldehydes and ketones, carbon of carbonyl group is [MP PMT 1995; RPET 1999, 2000]
\( >C=O ) \) (sp2-hybridized)
Question#13
The non-carbonyl compound out of the following is ; (2018-ETEA)
Question#14
HCOOH is the structure of NUMS2018
Question#15
Which one of the following is IUPAC name of the above given structure ? MDCAT2016
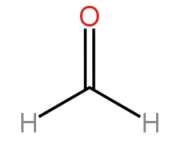
Question#16
Both aldehyde and ketone are planer to the neighborhoods of the carbonyl (>C=O) group. Which one of the following bond is distorted toward oxygen atom MDCAT2015
Question#17
Which of following is the structure of a ketone MDCAT2013
Question#18
Which of the following compound belong to homologous series of aldehydes MDCAT2011
Question#19
The common name of following aldehyde is MDCAT2020
Cl-CH2-CH-CHO
l
CH3