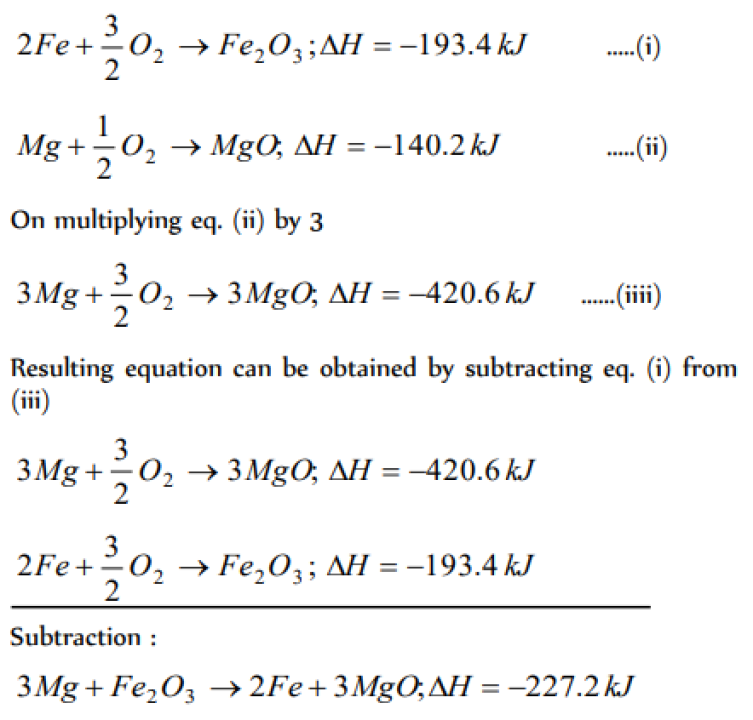
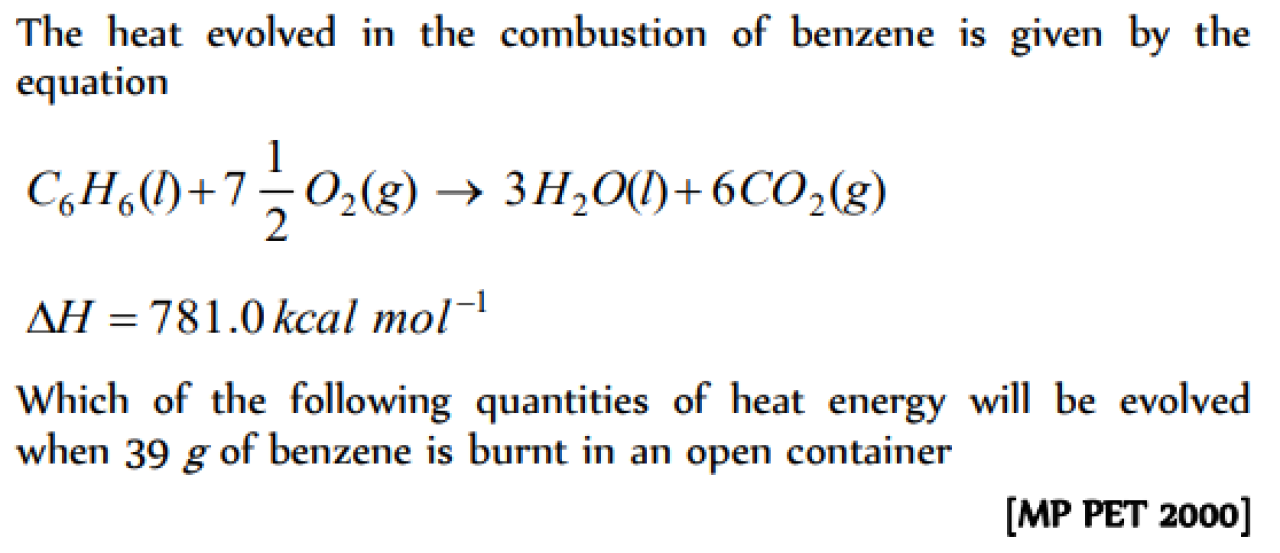
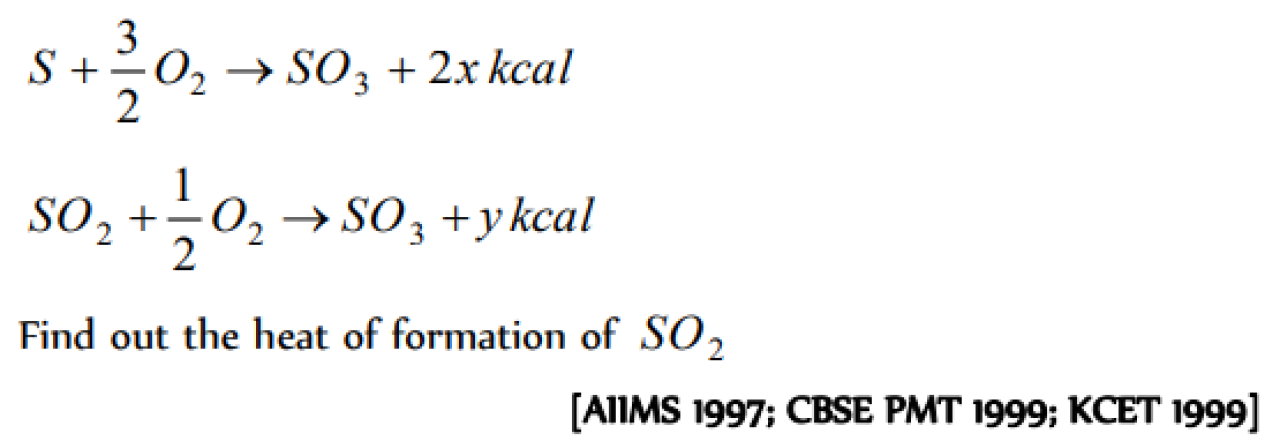
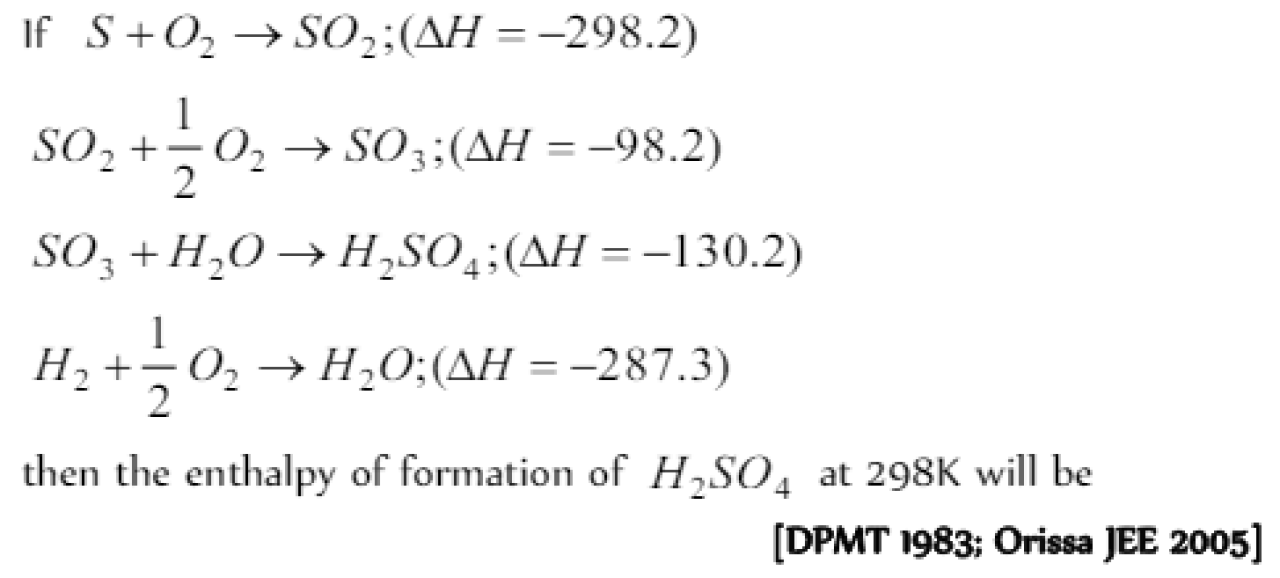
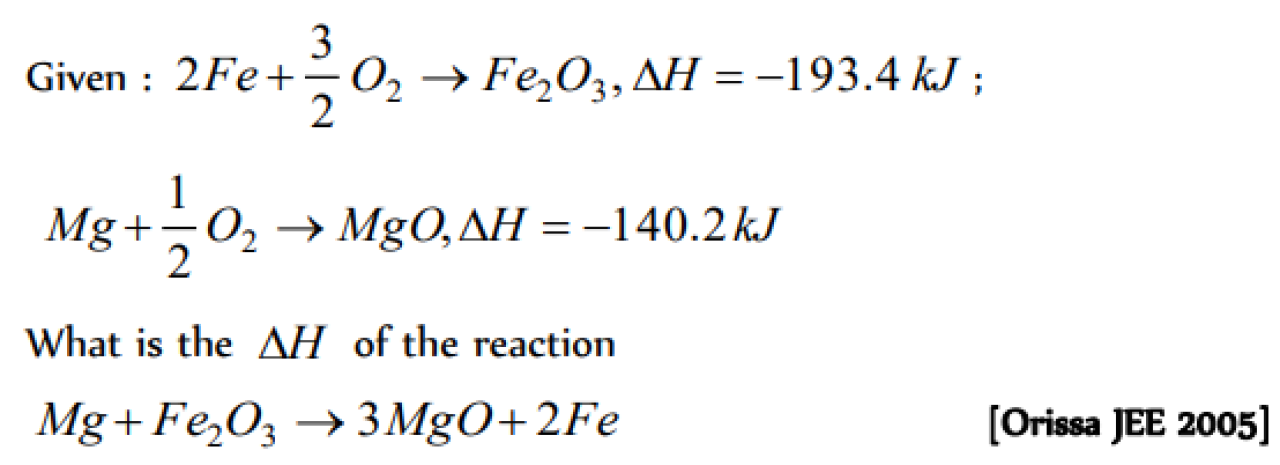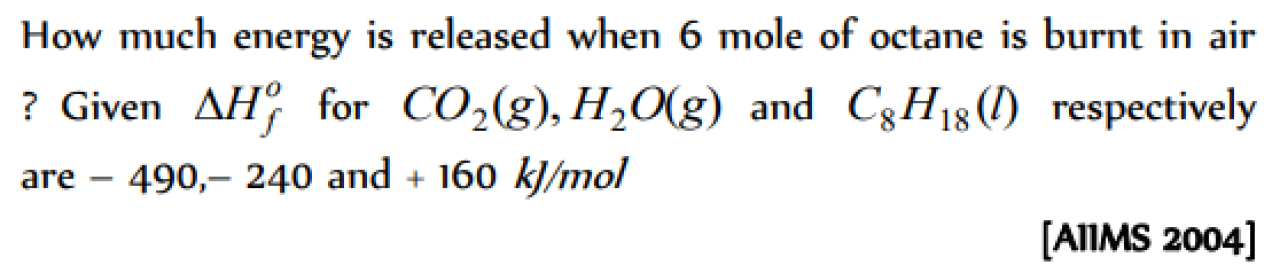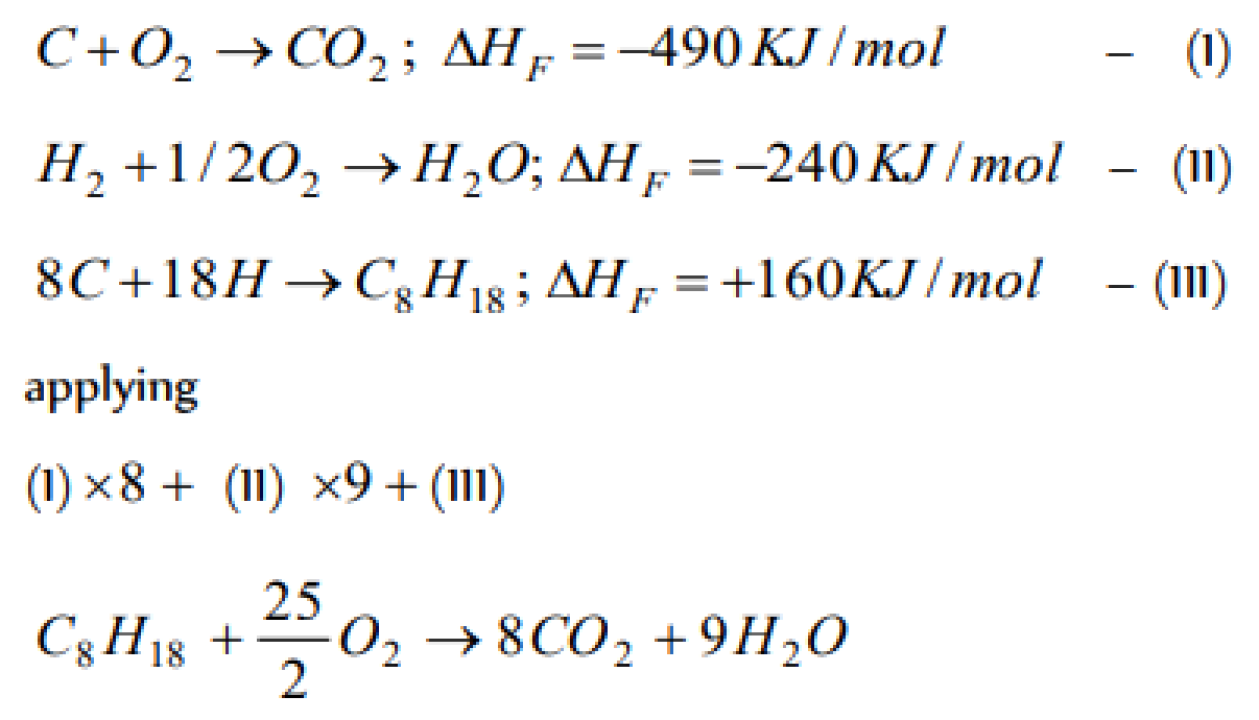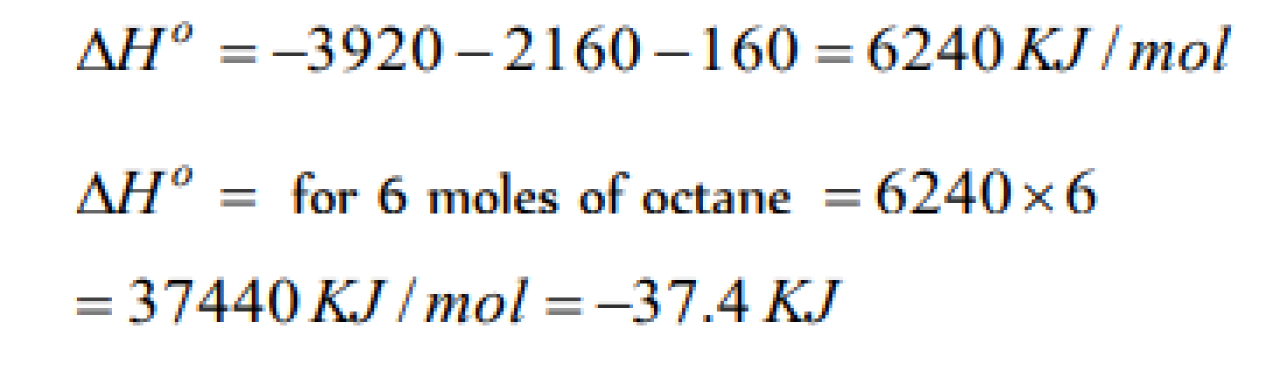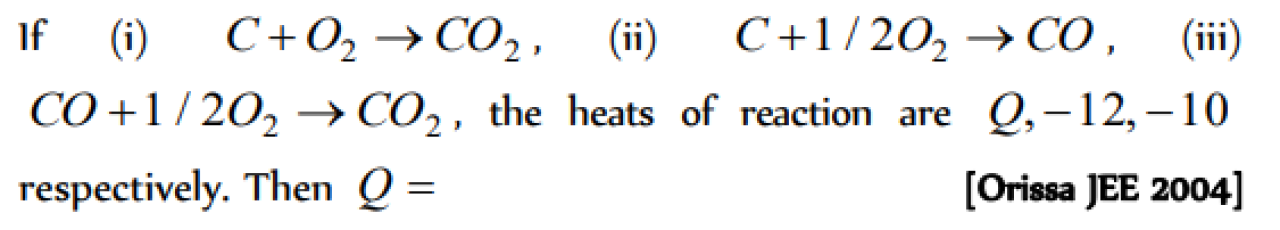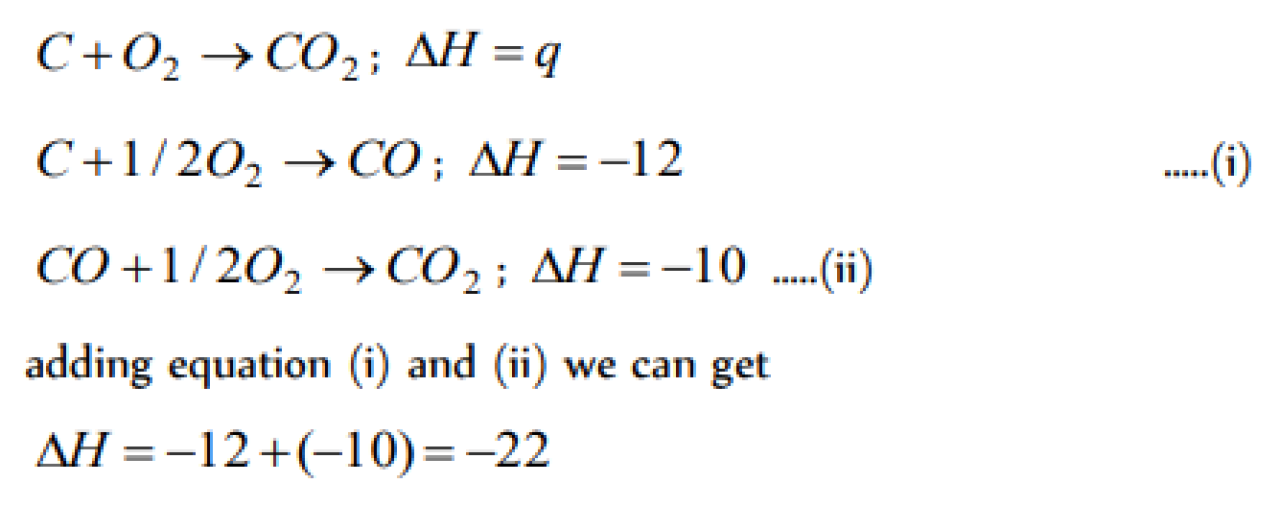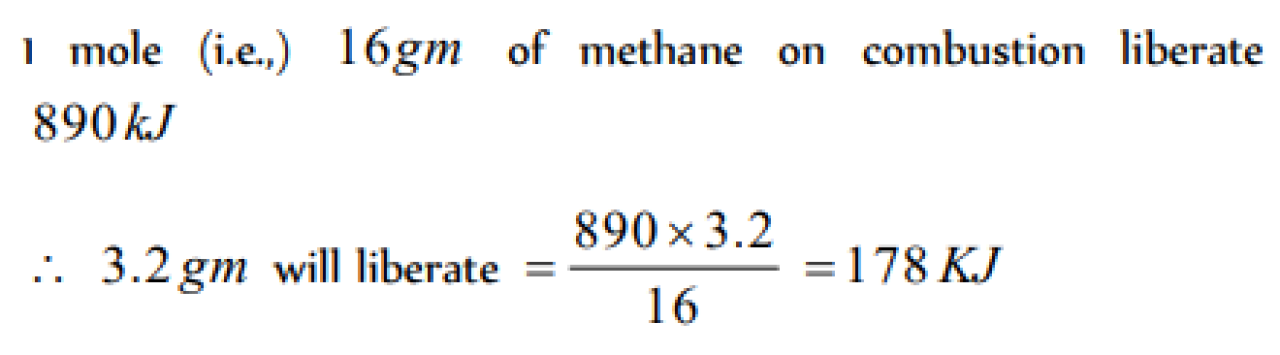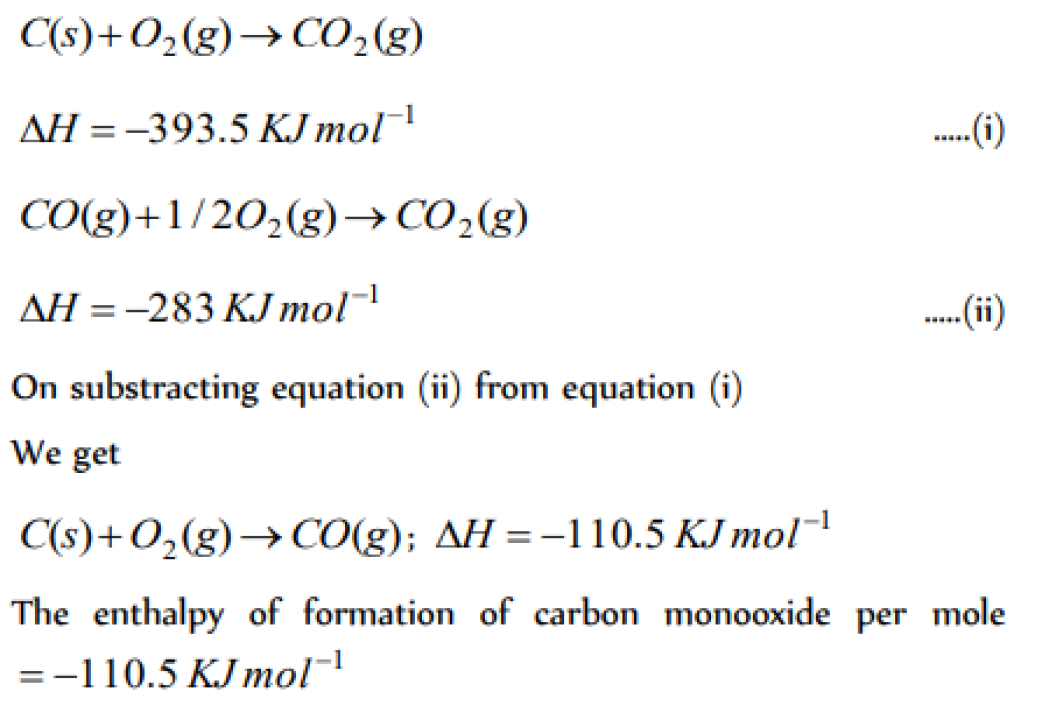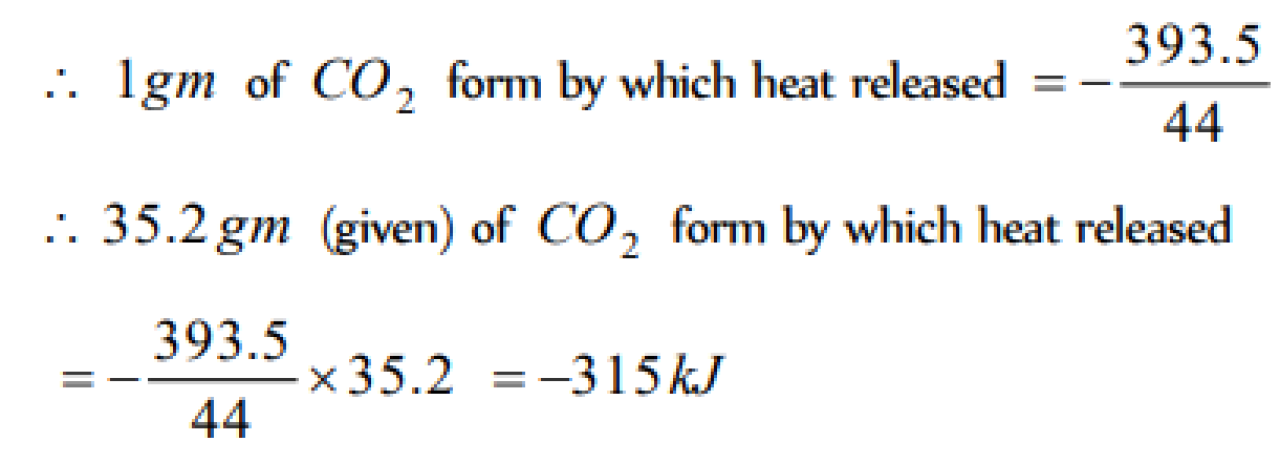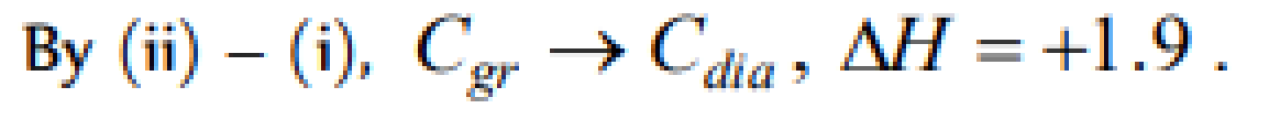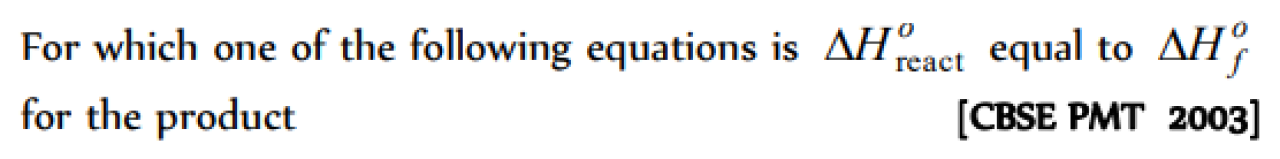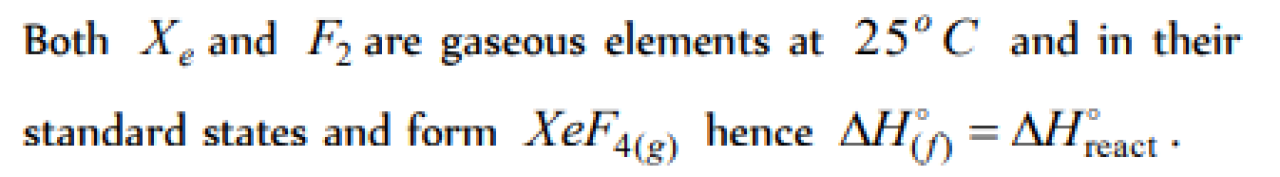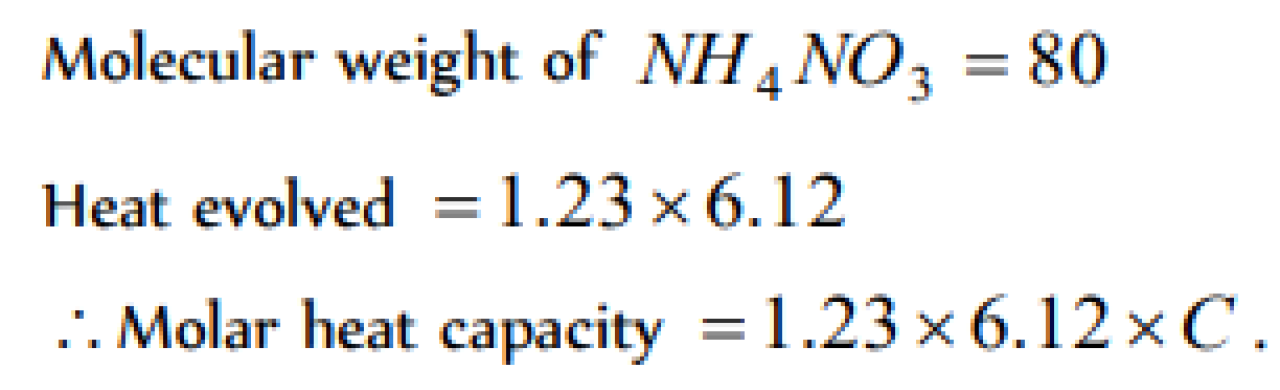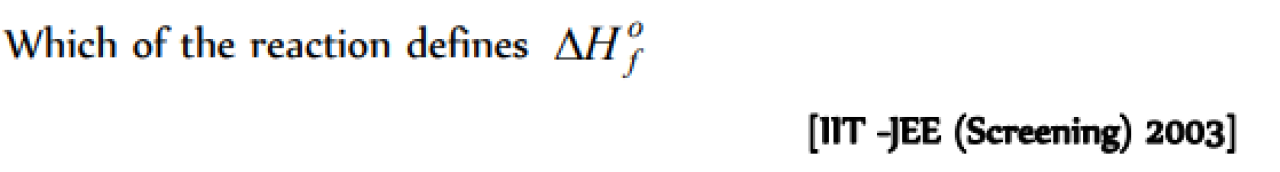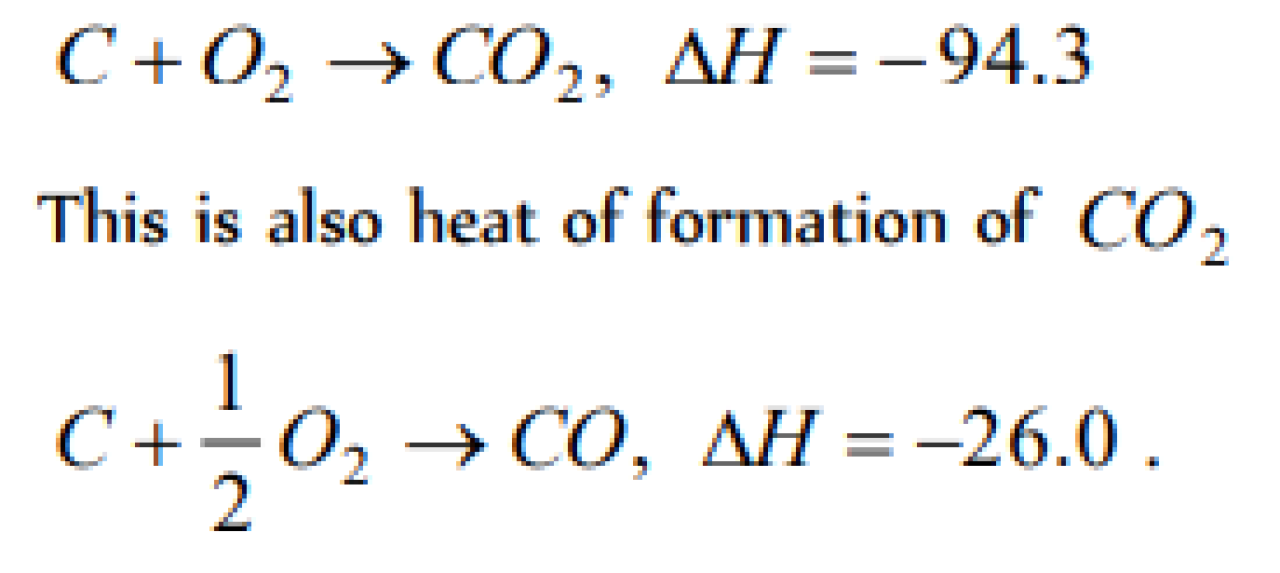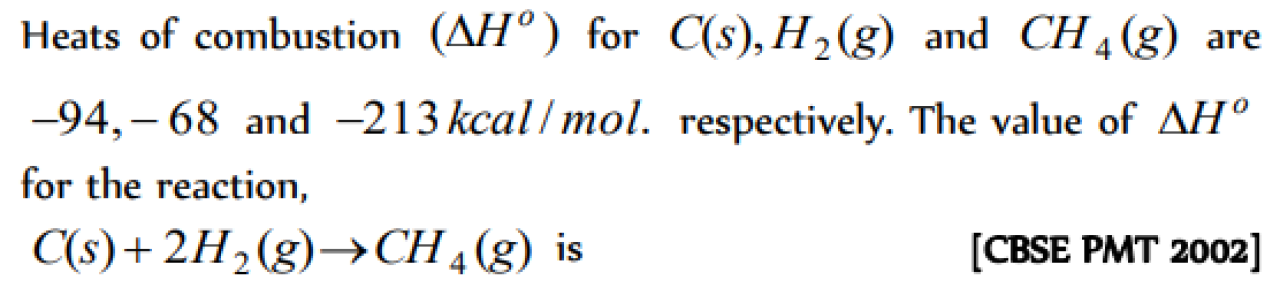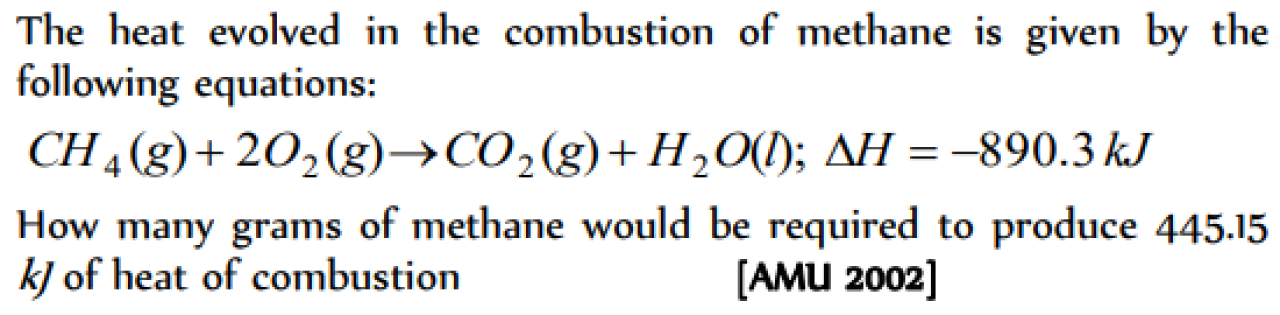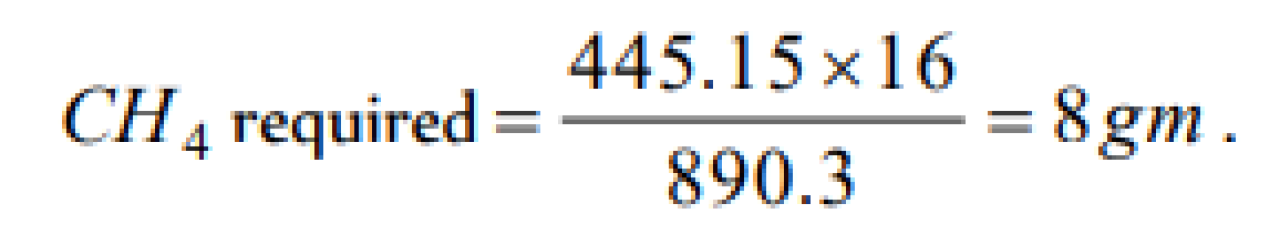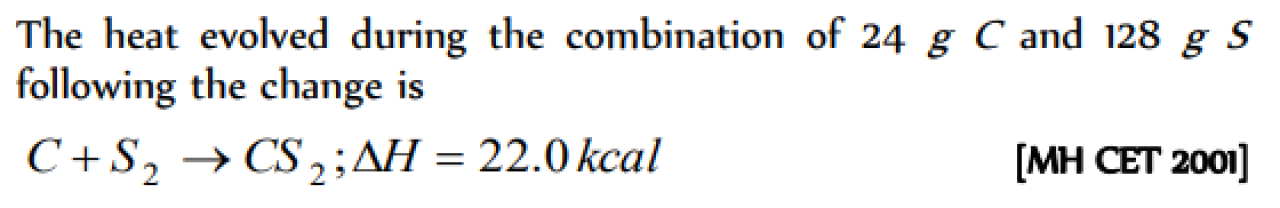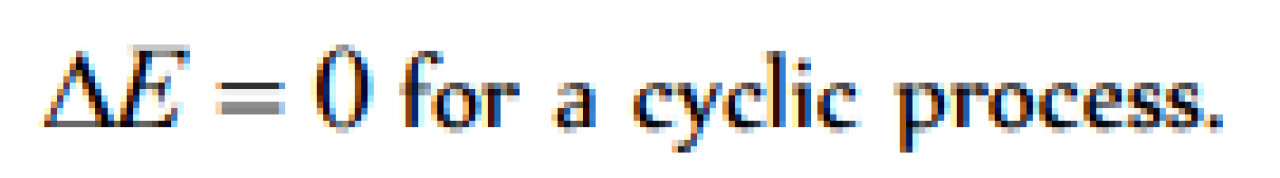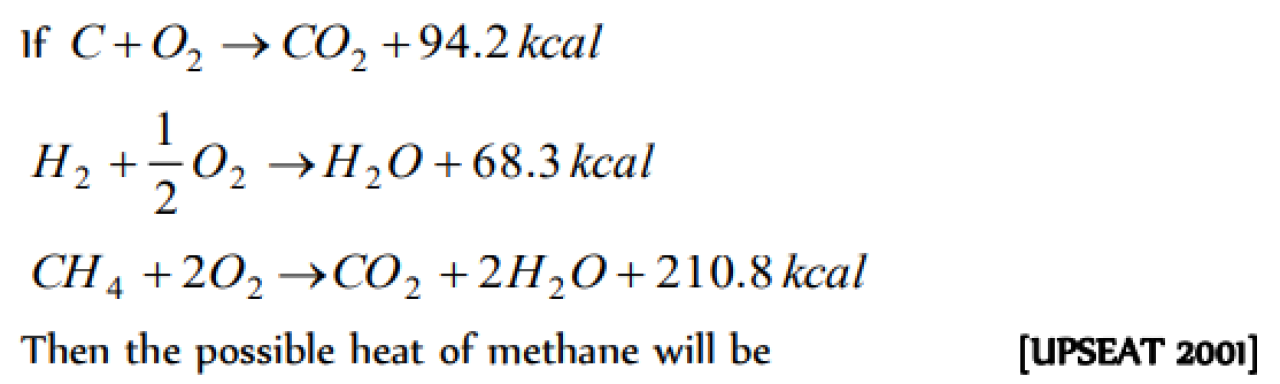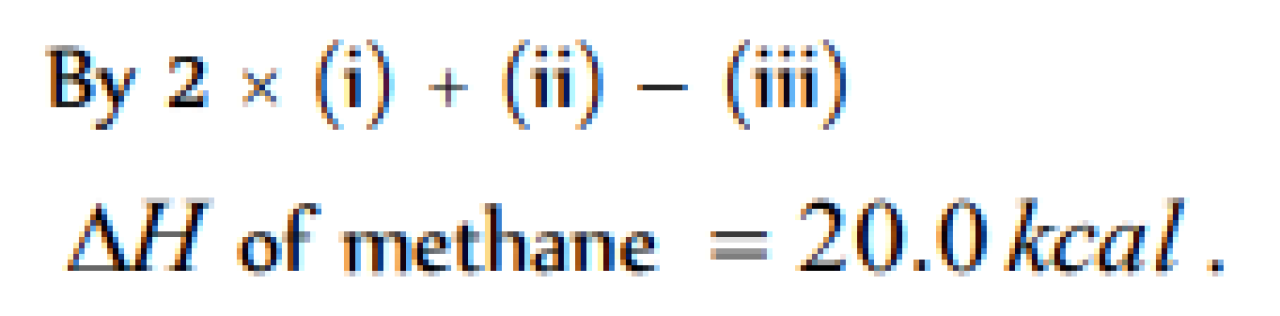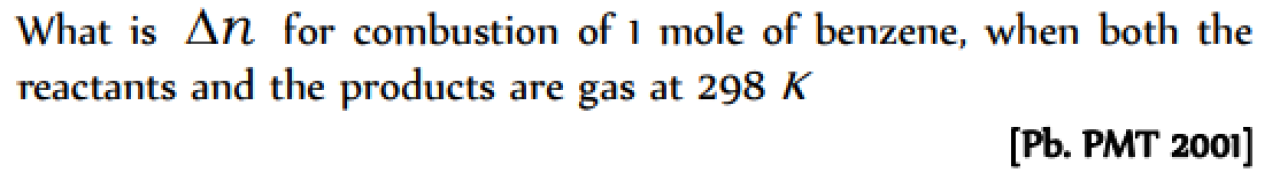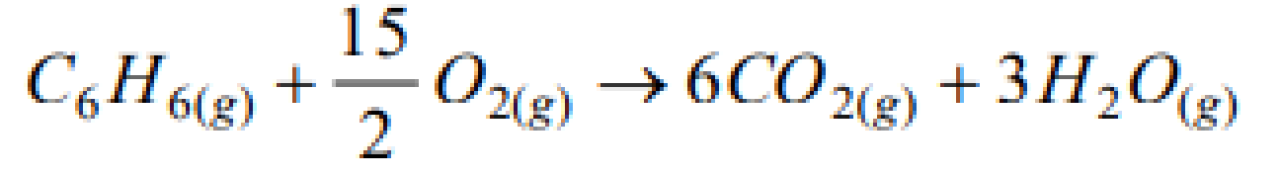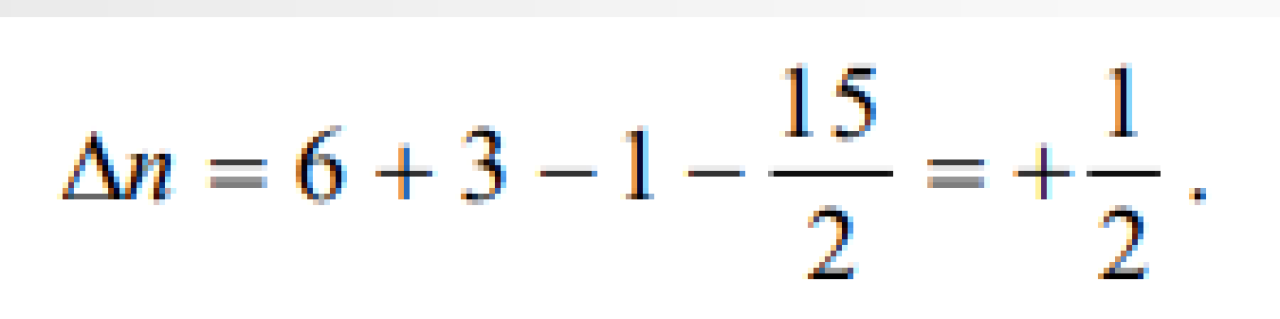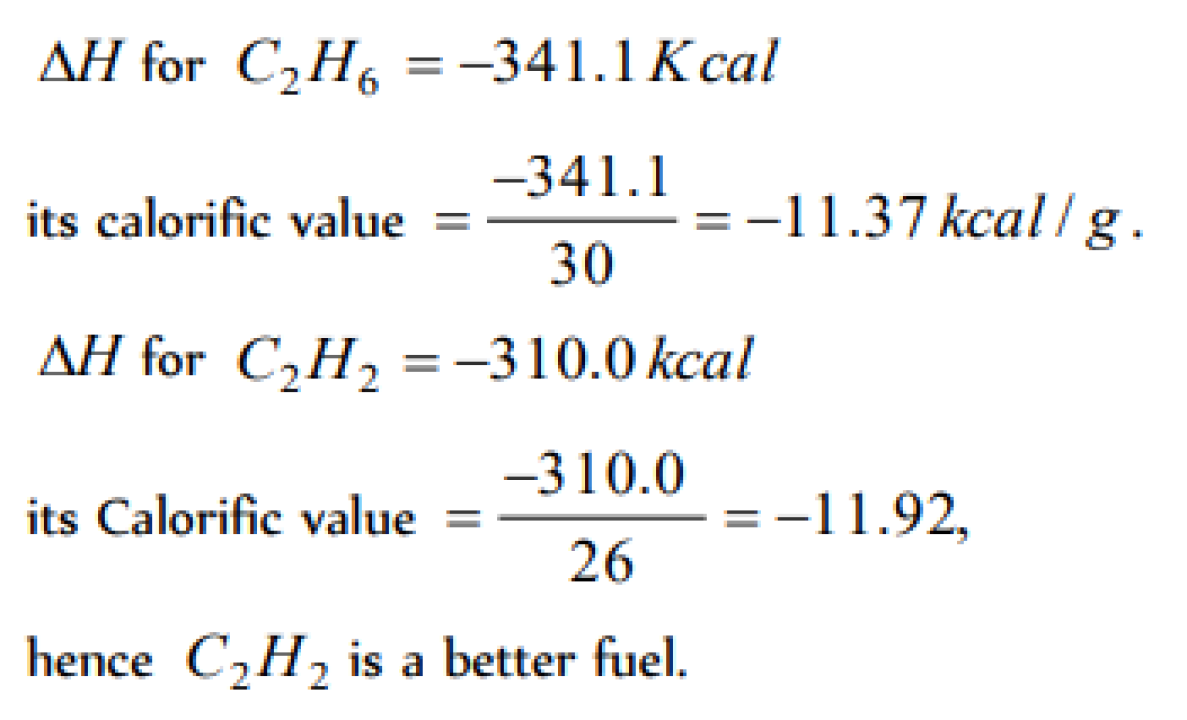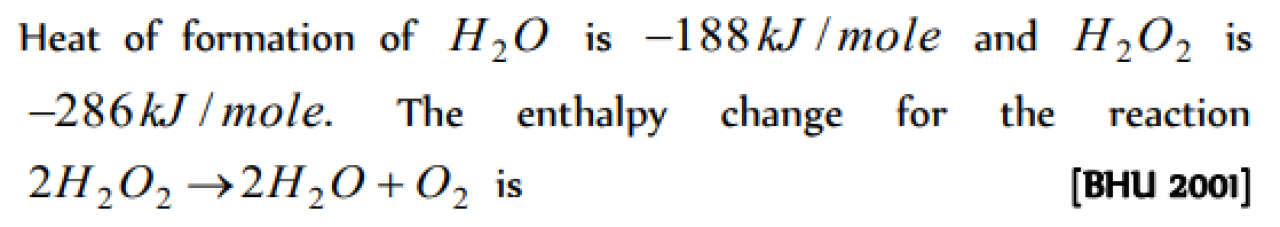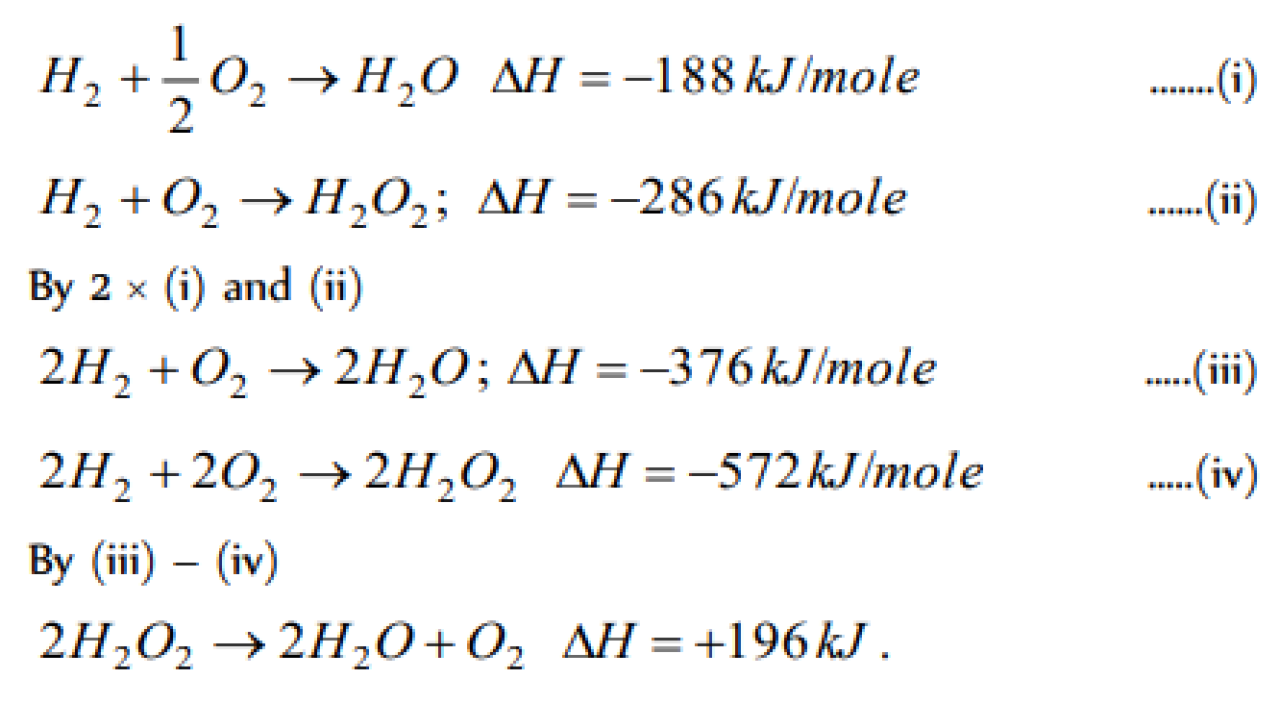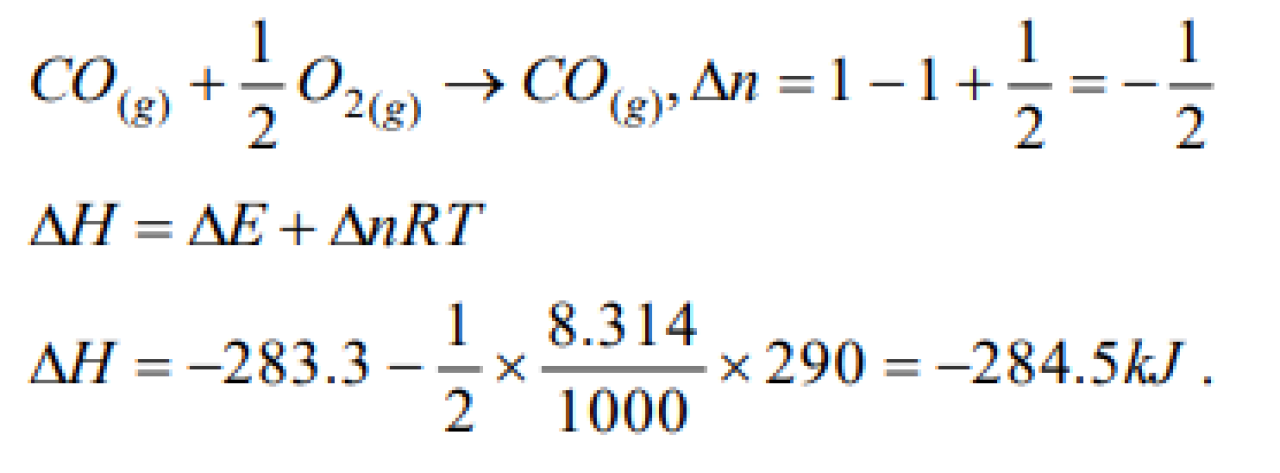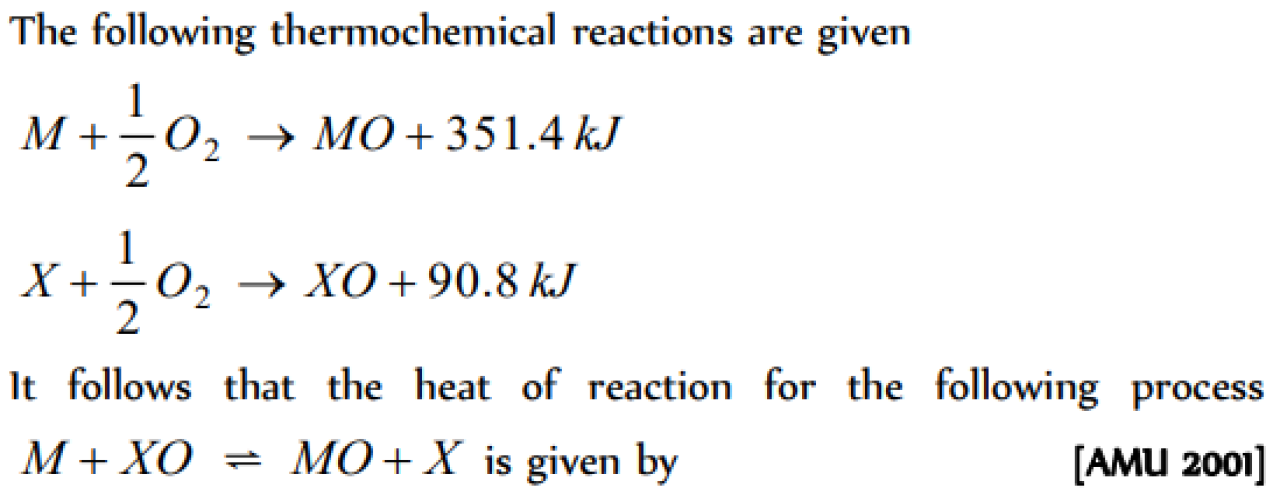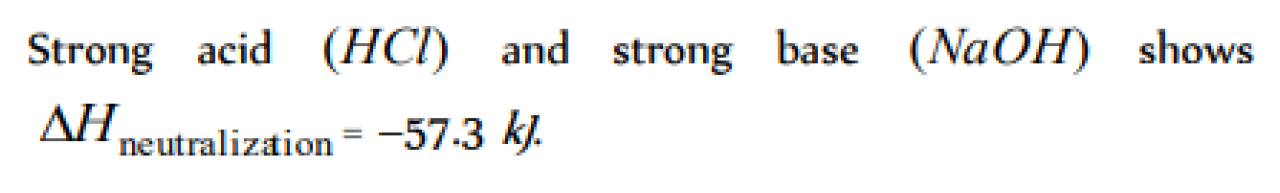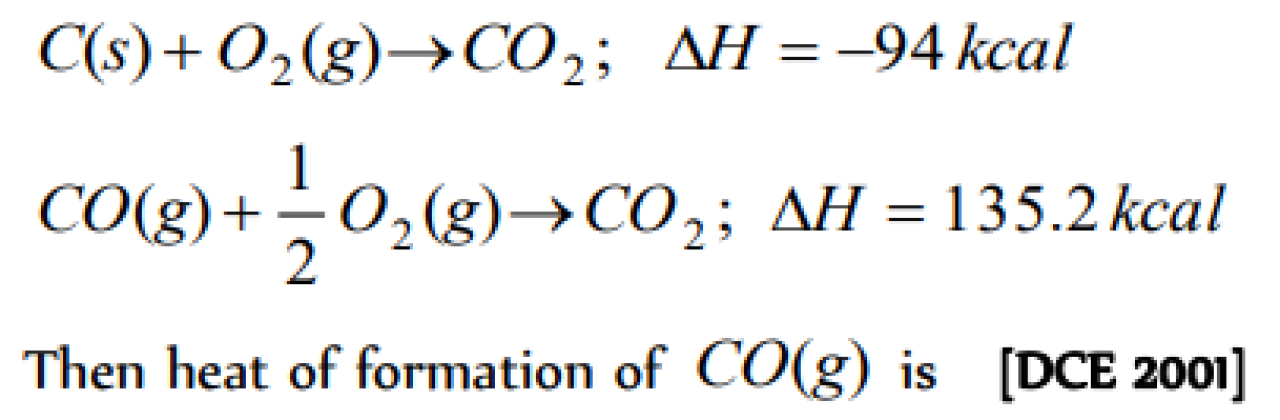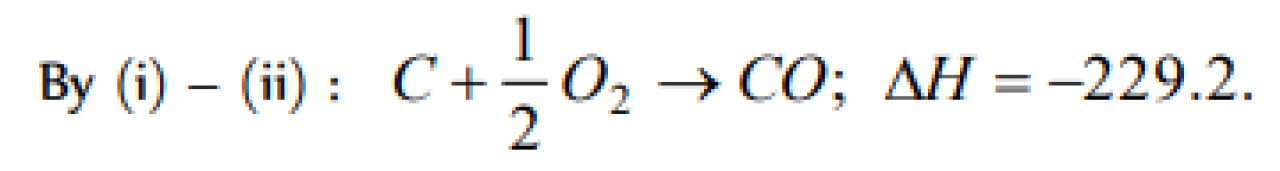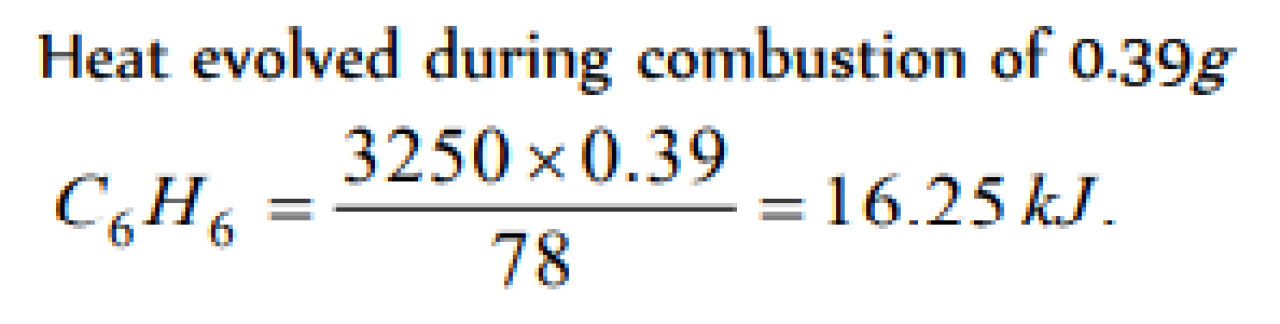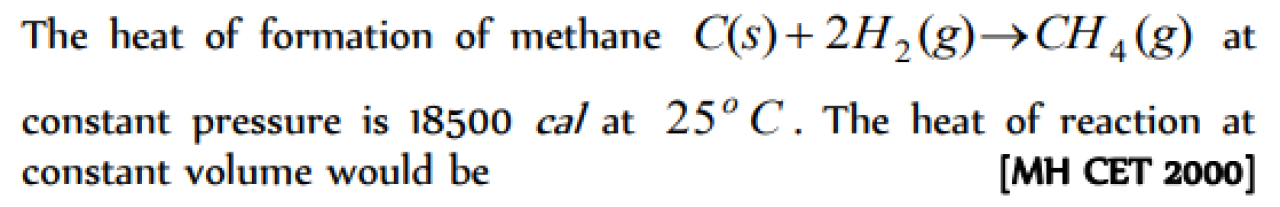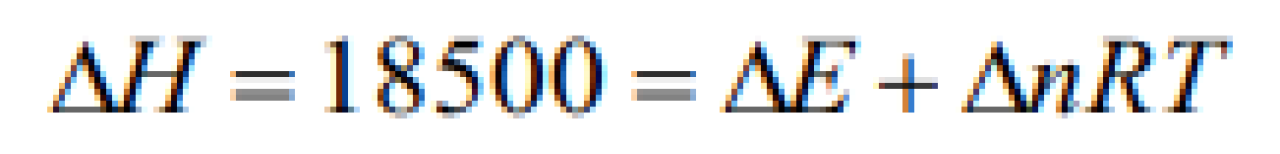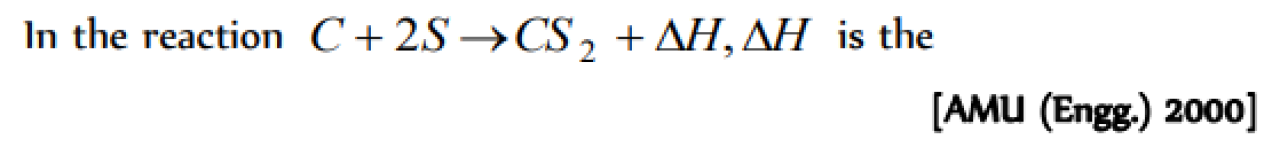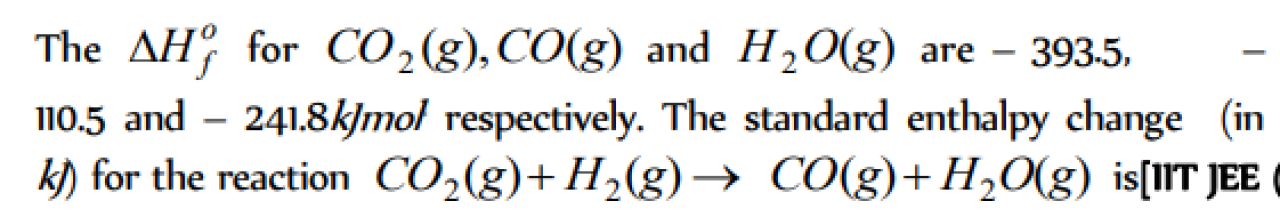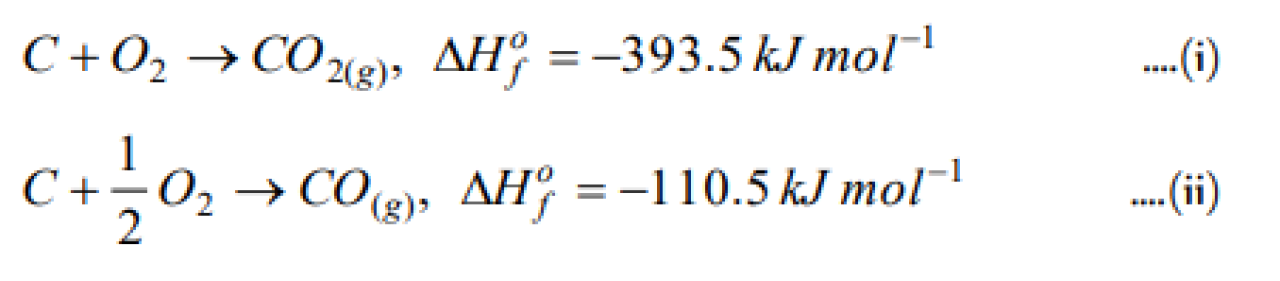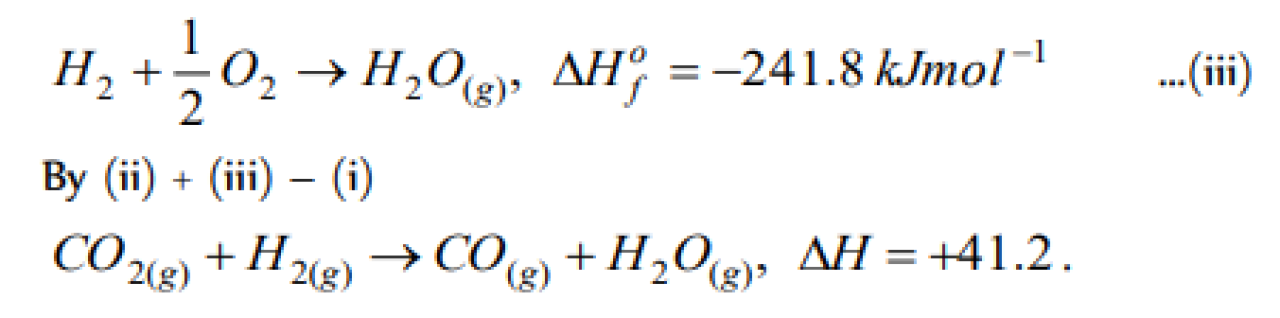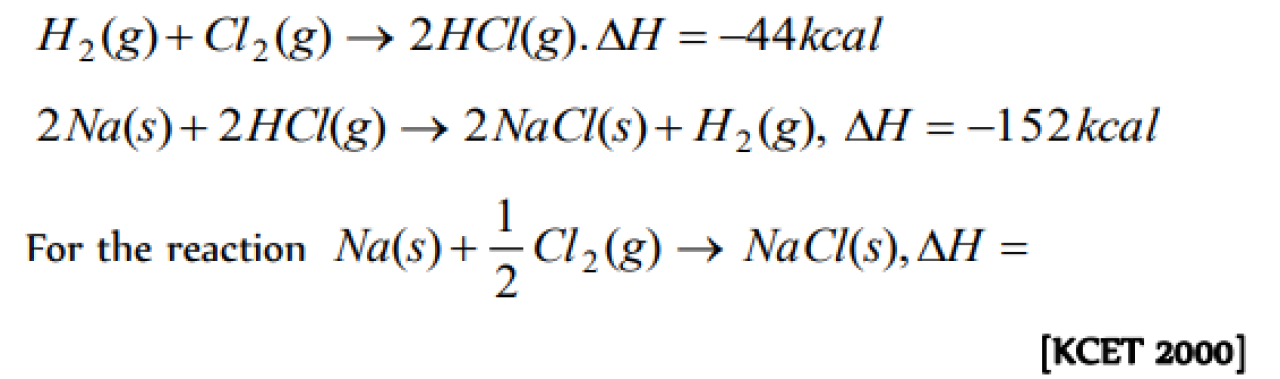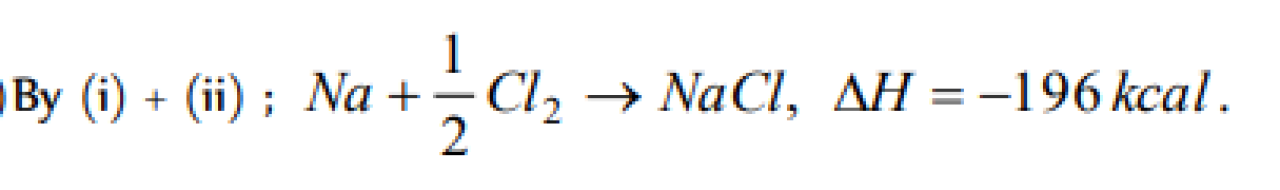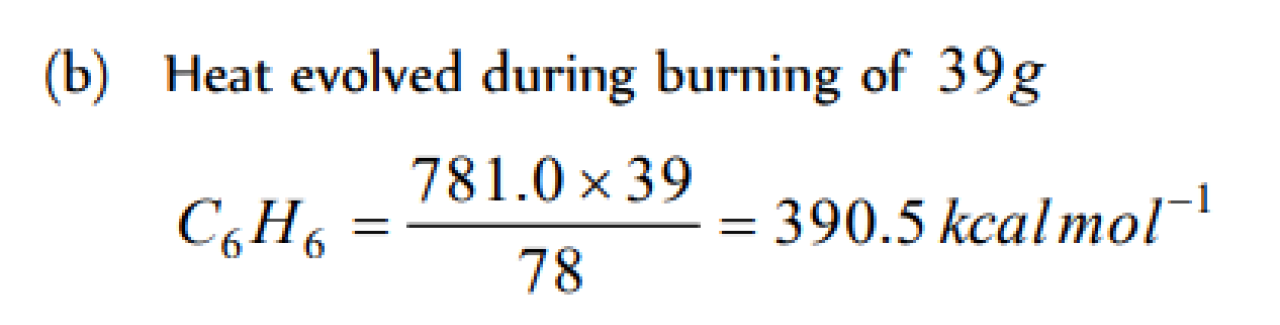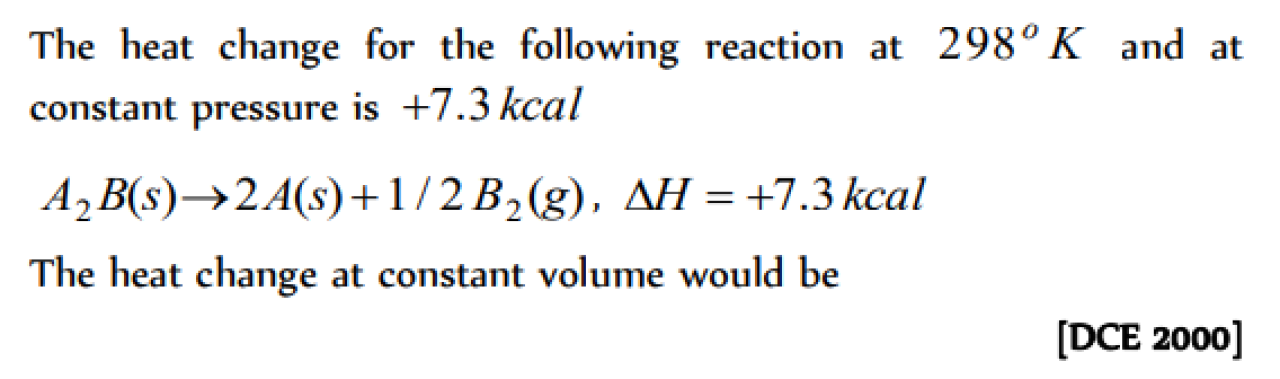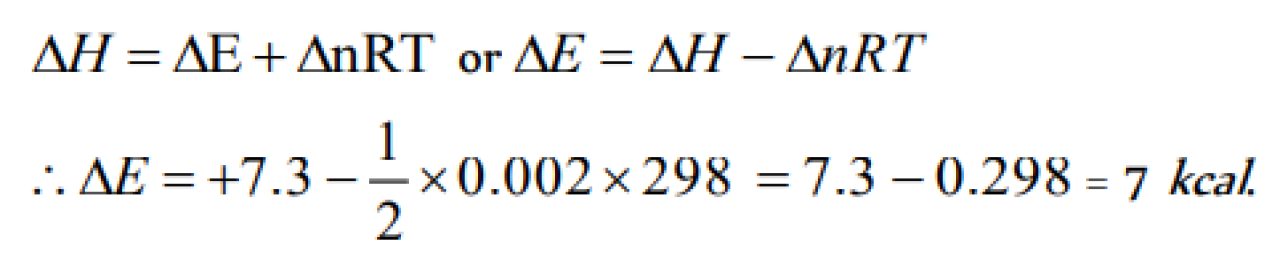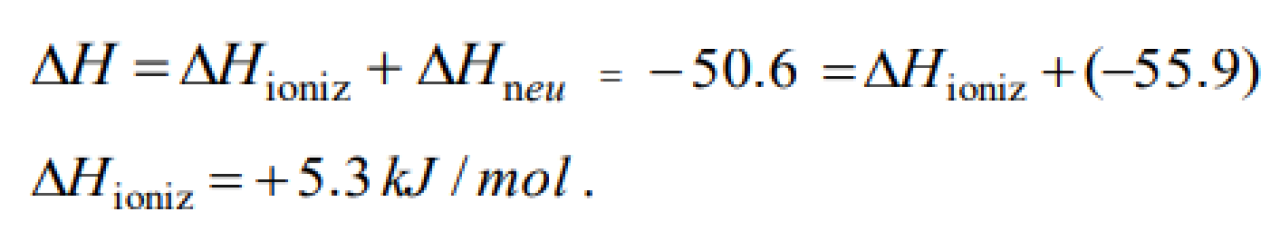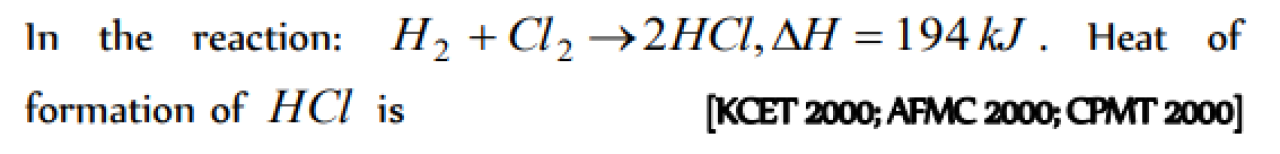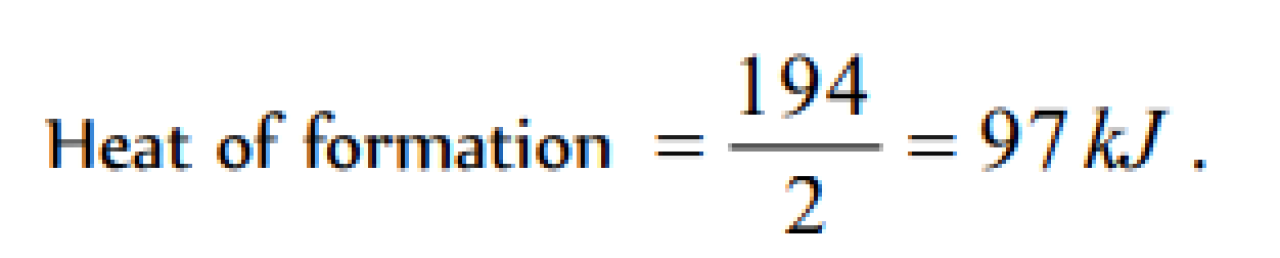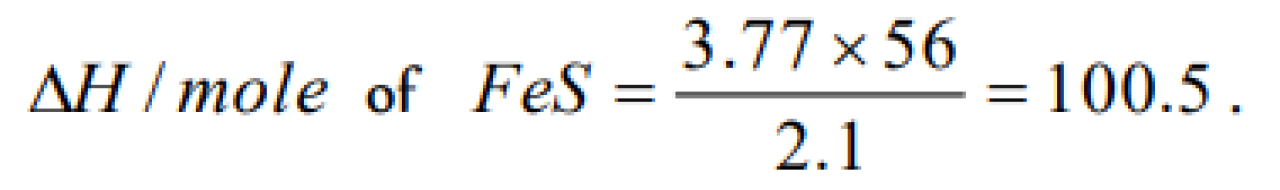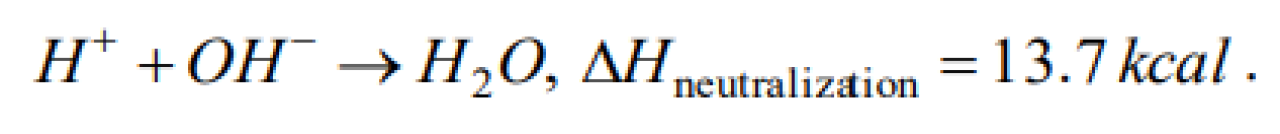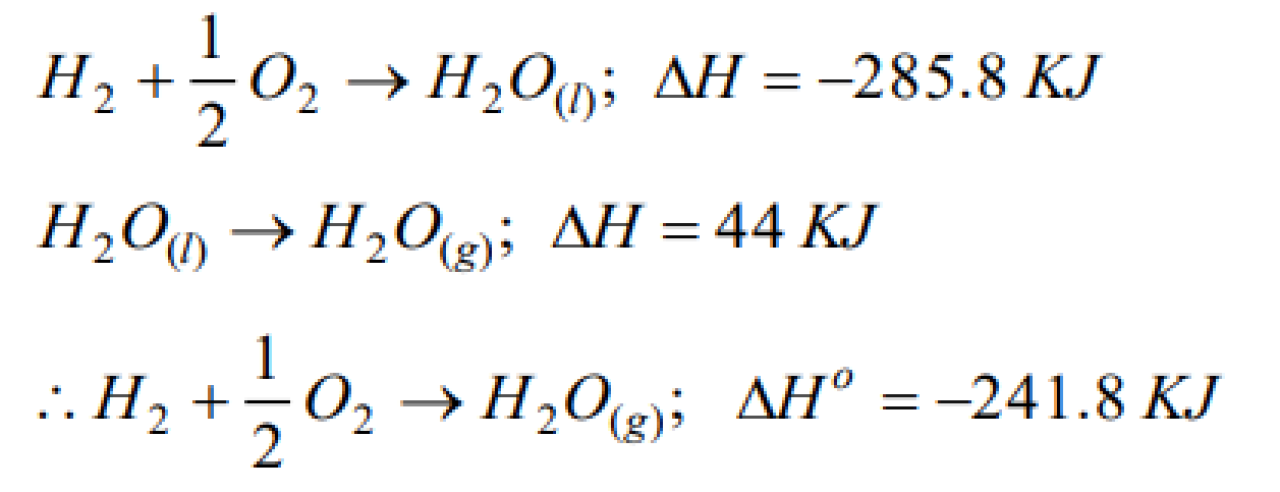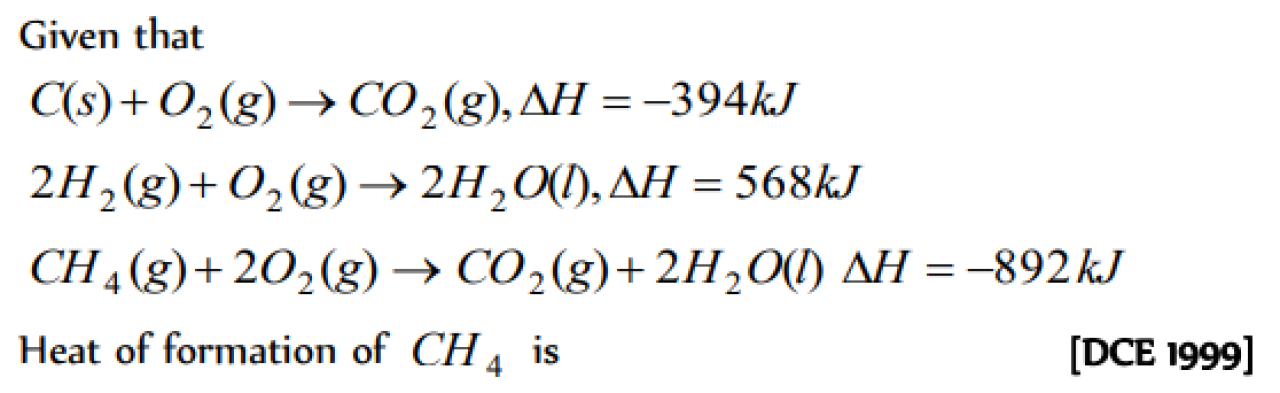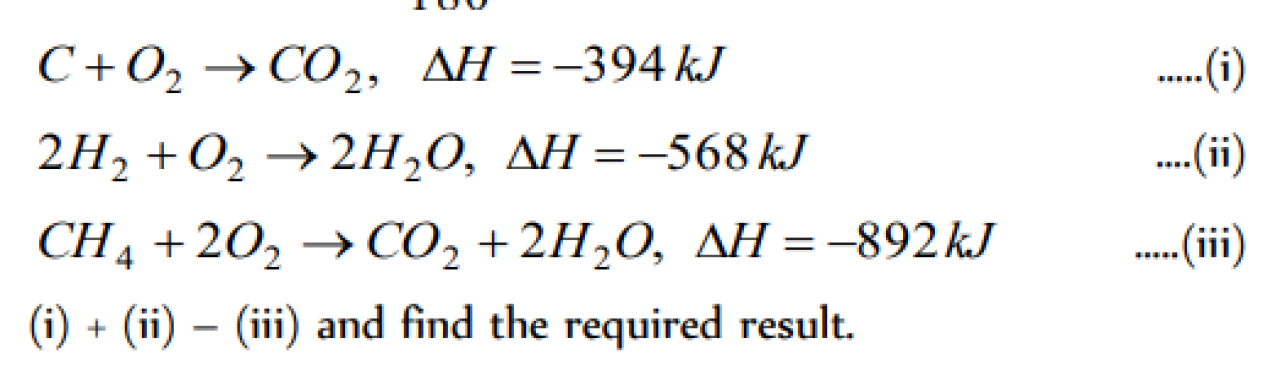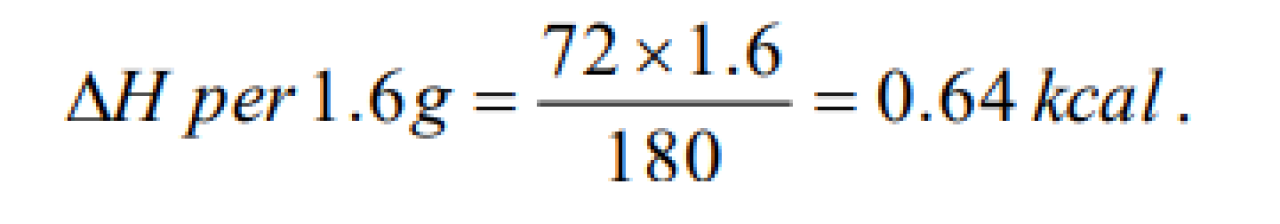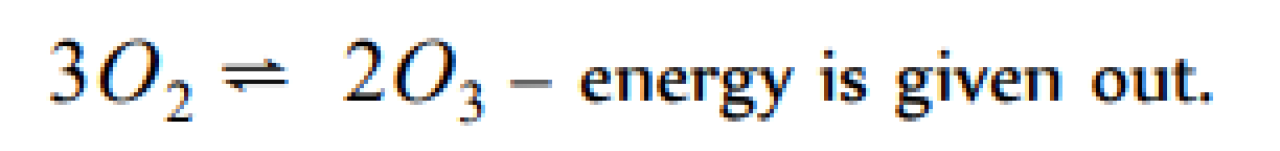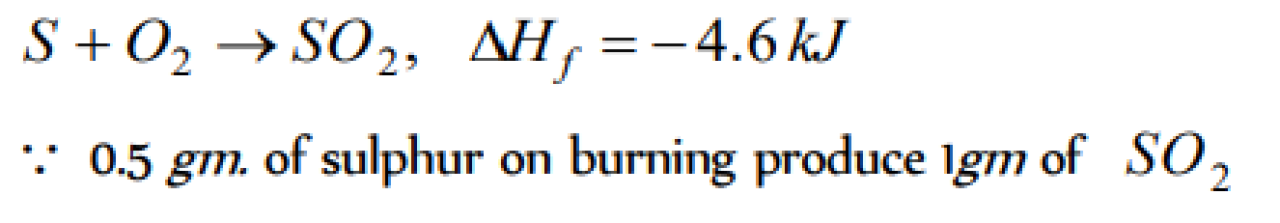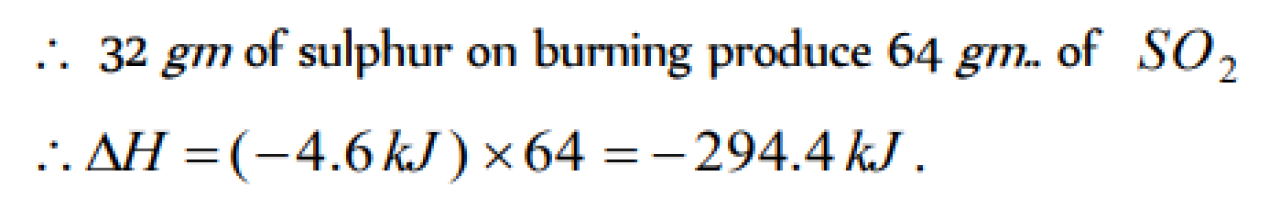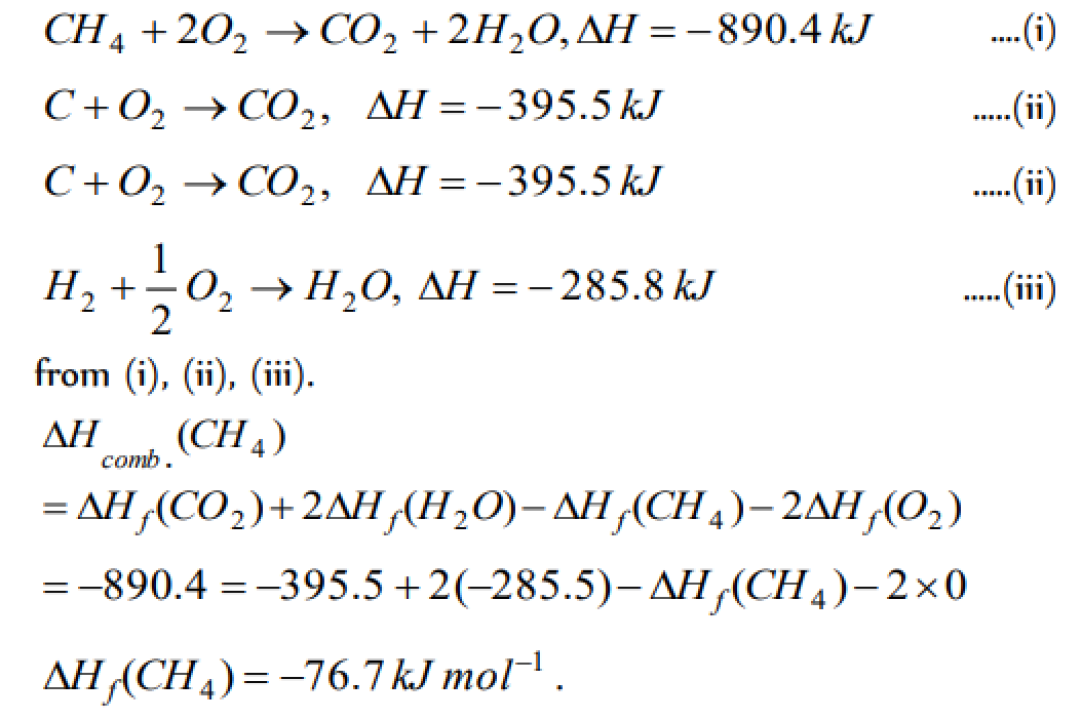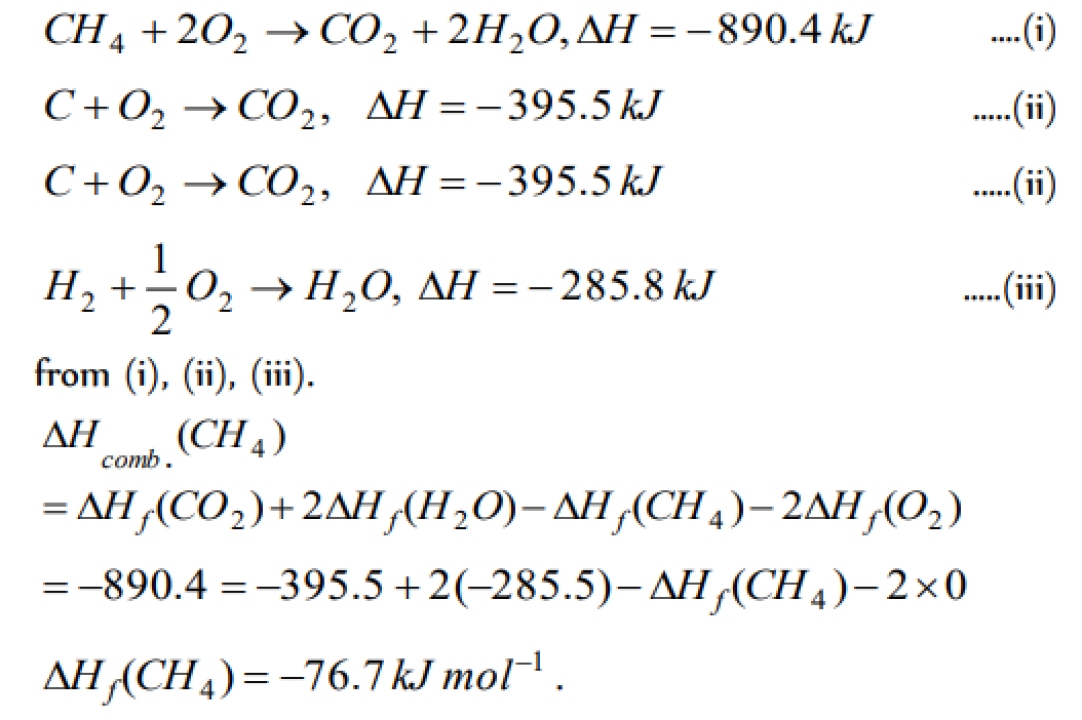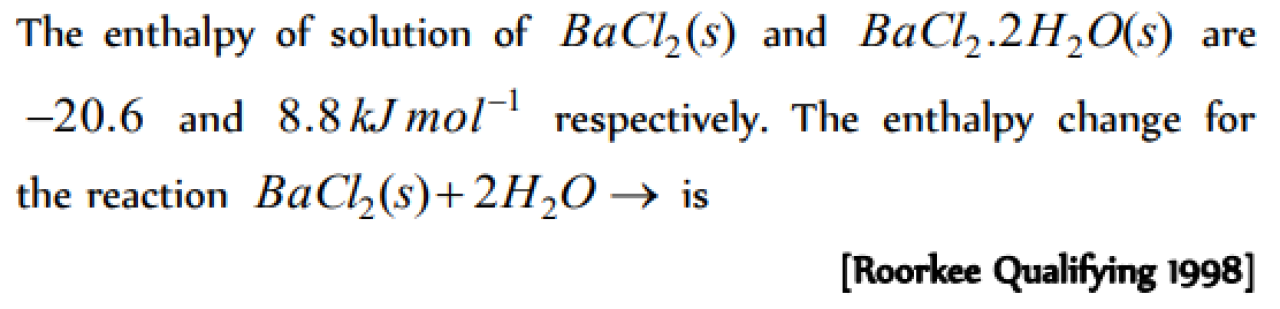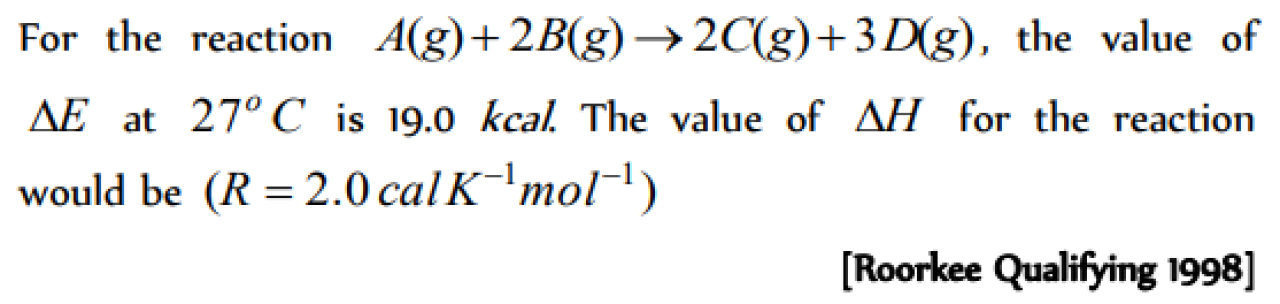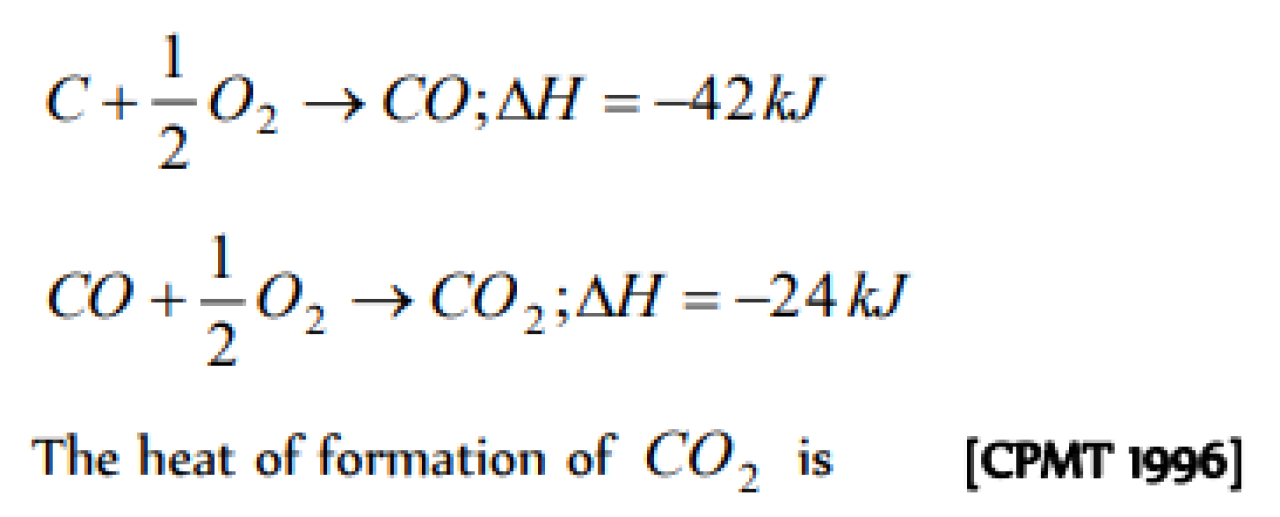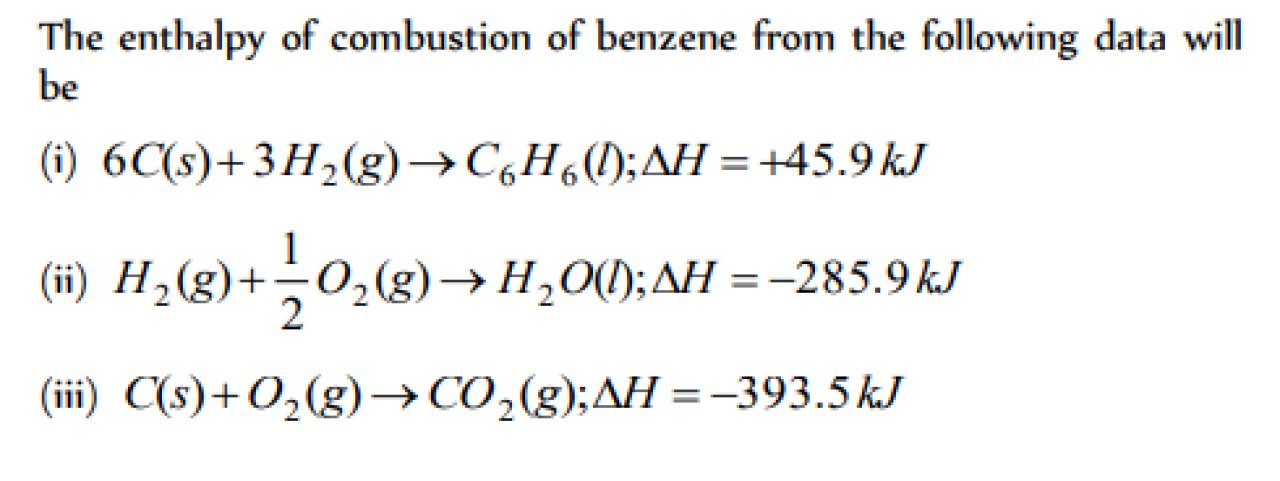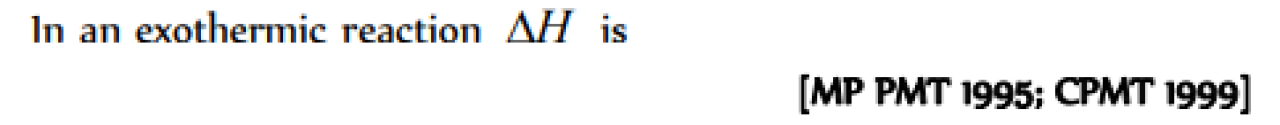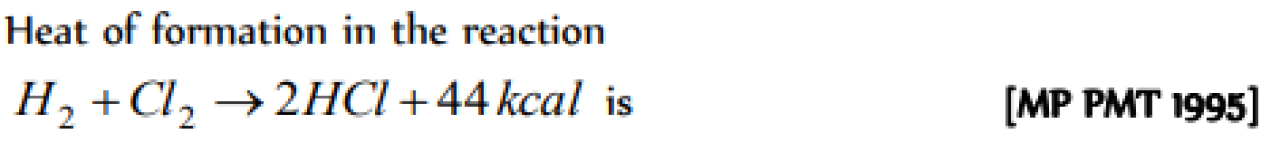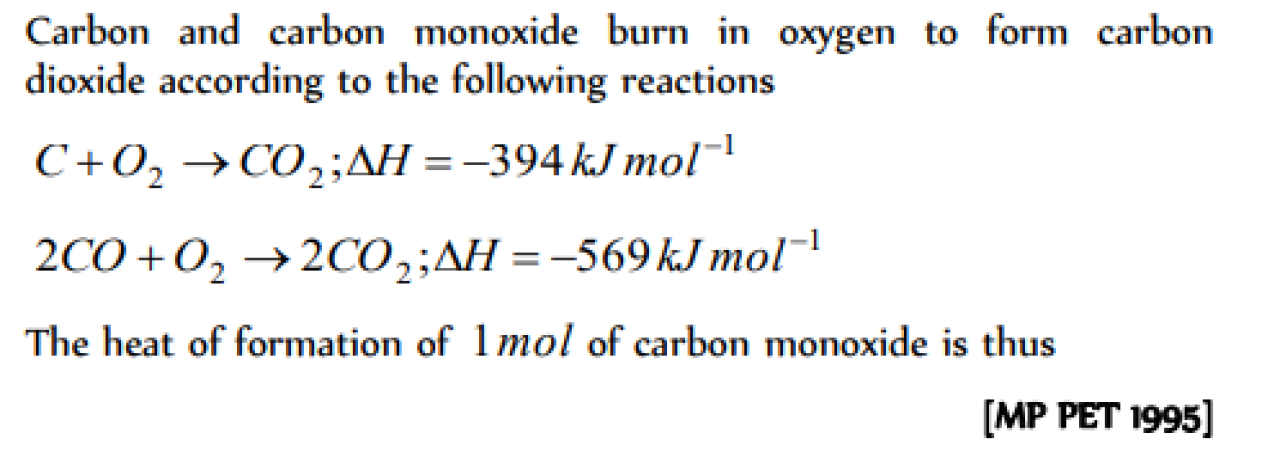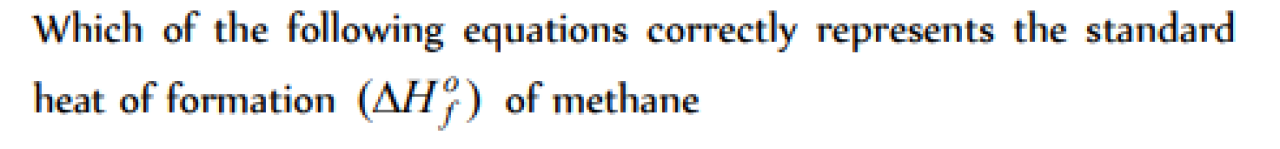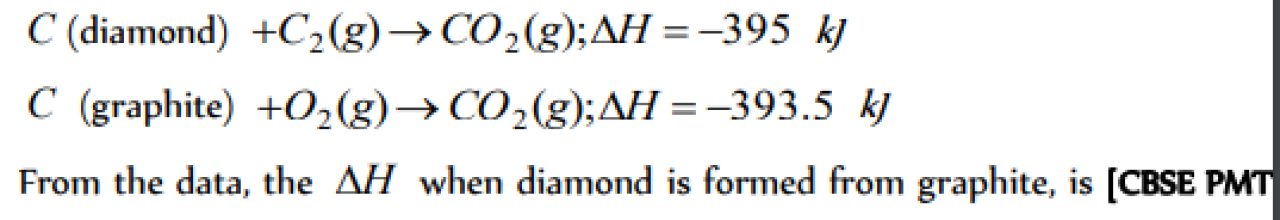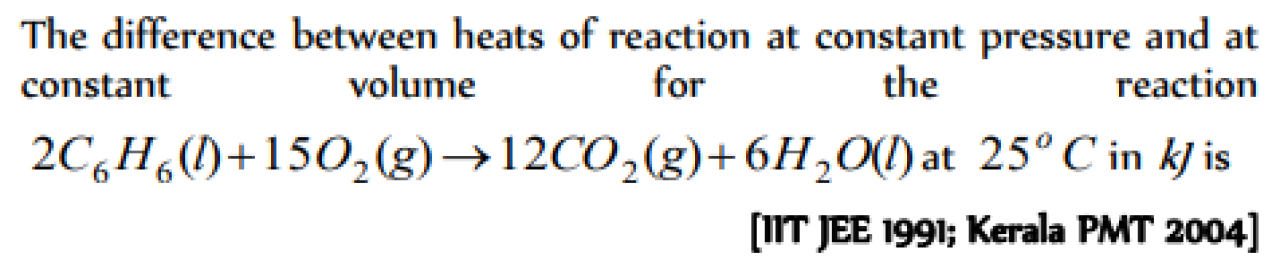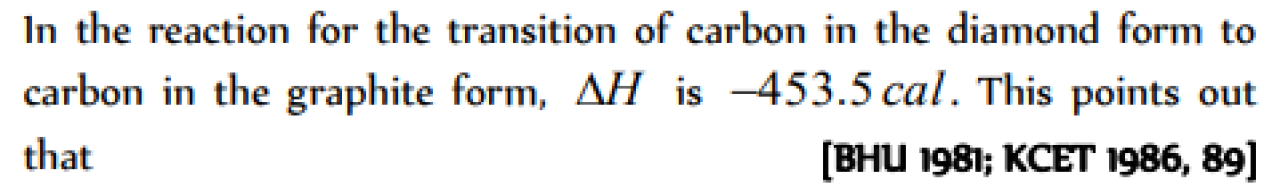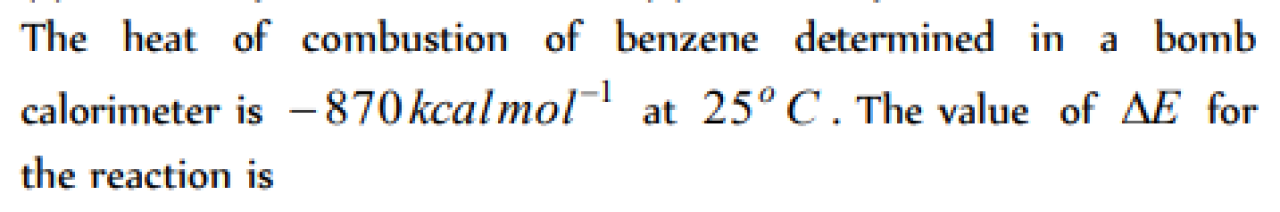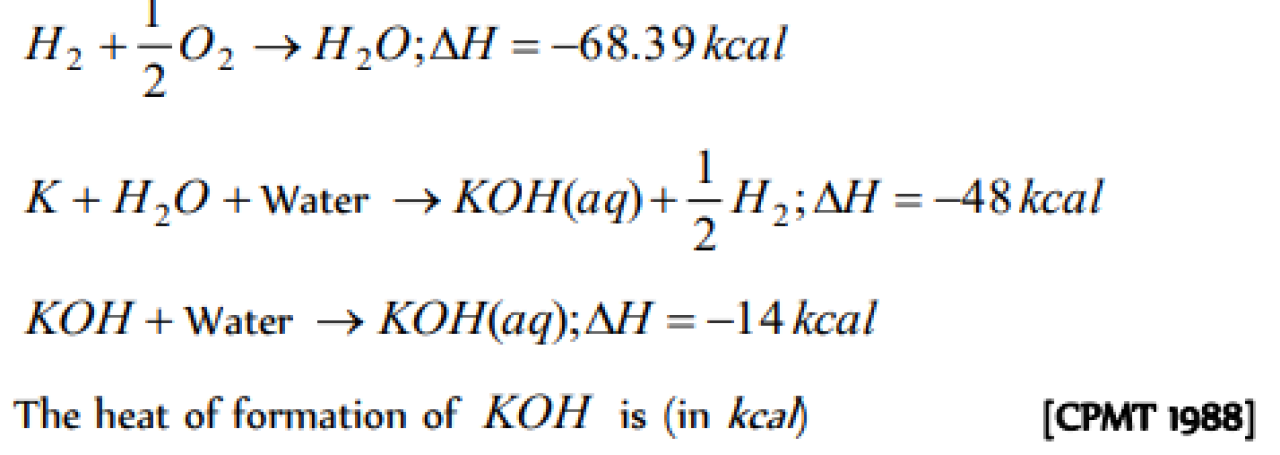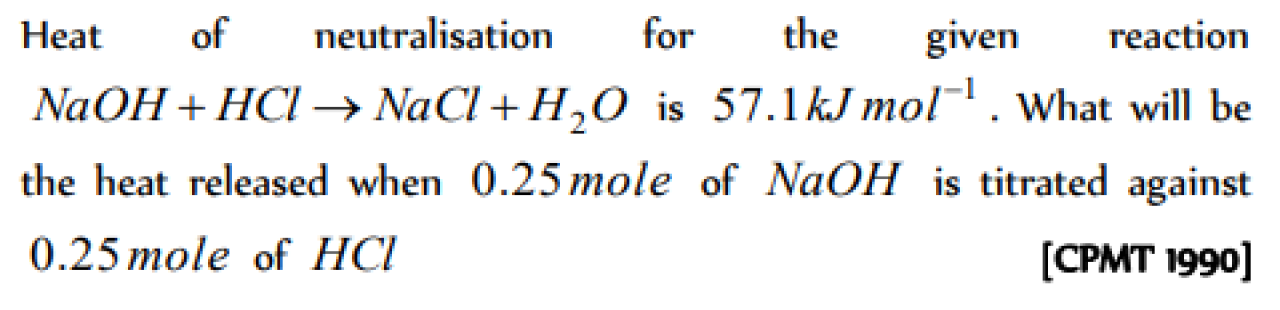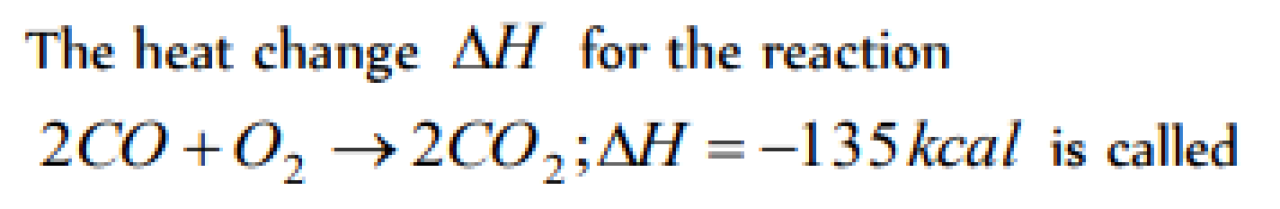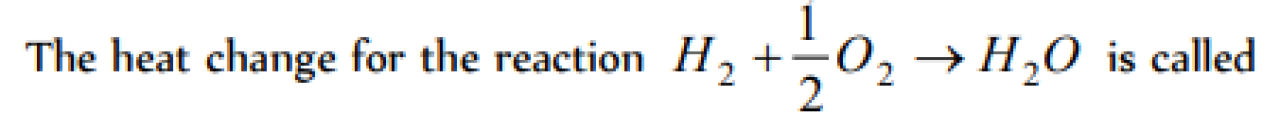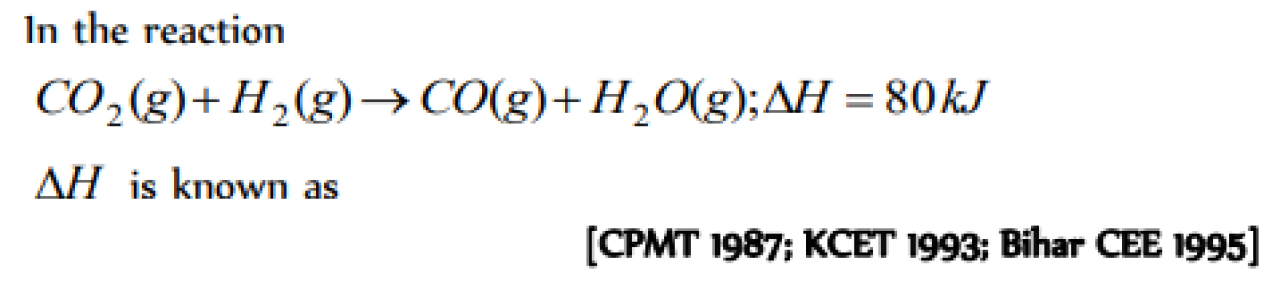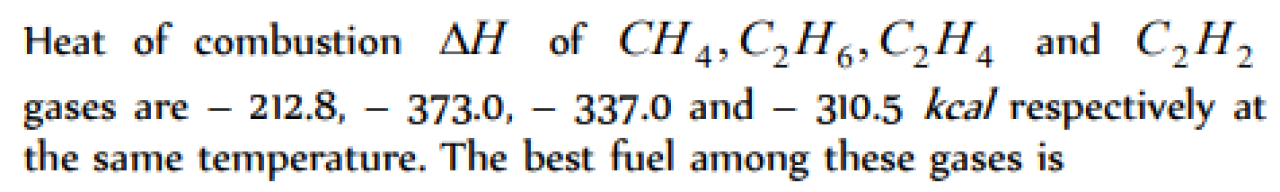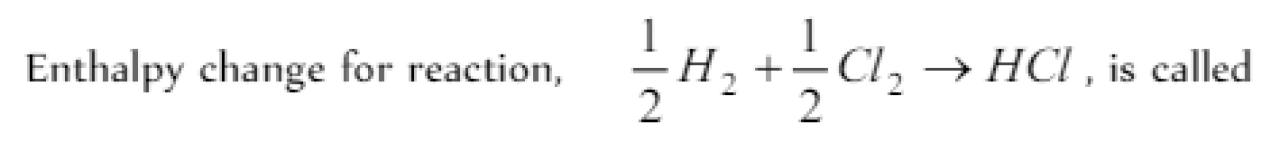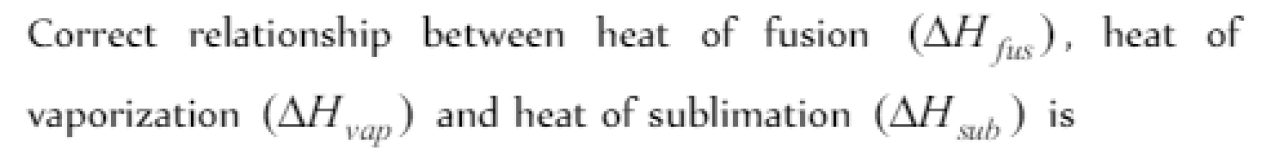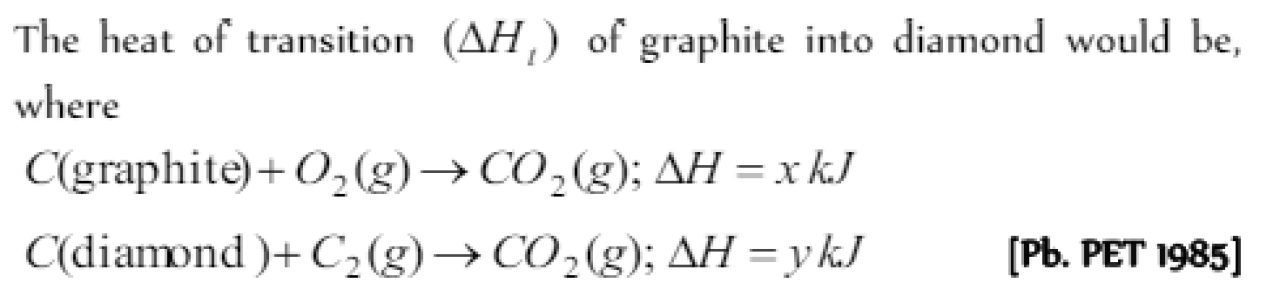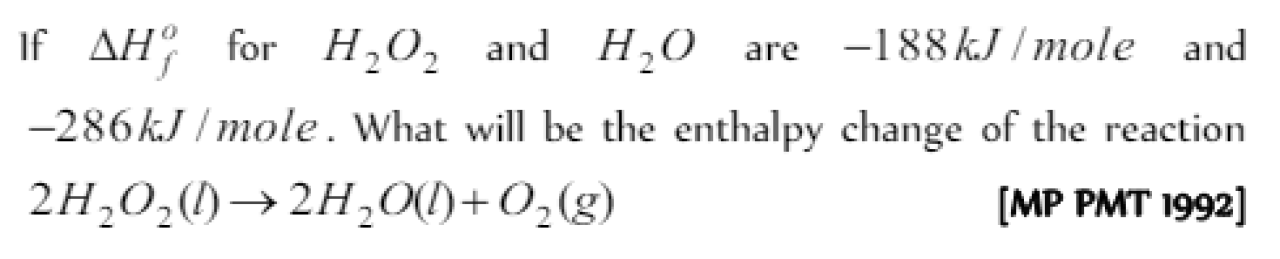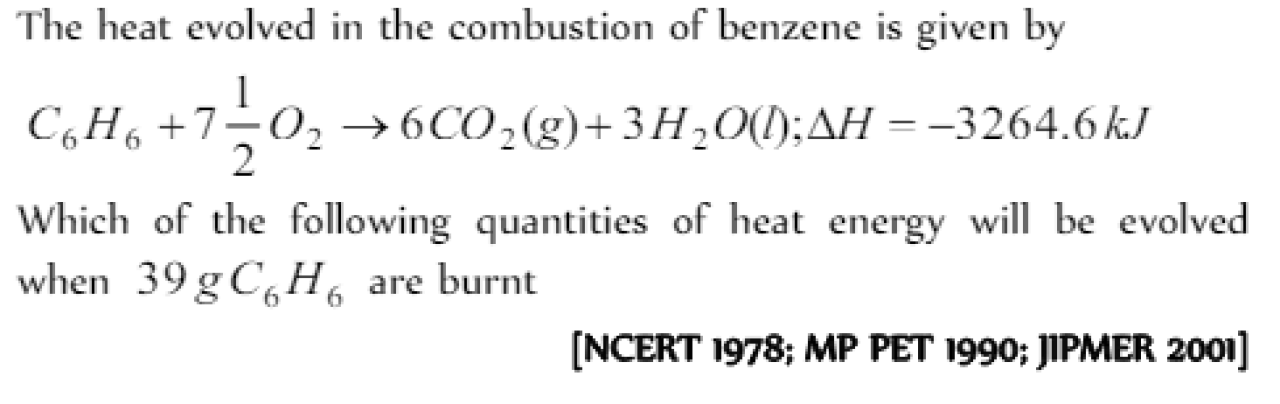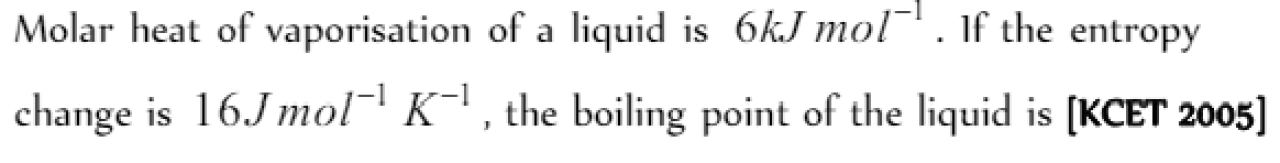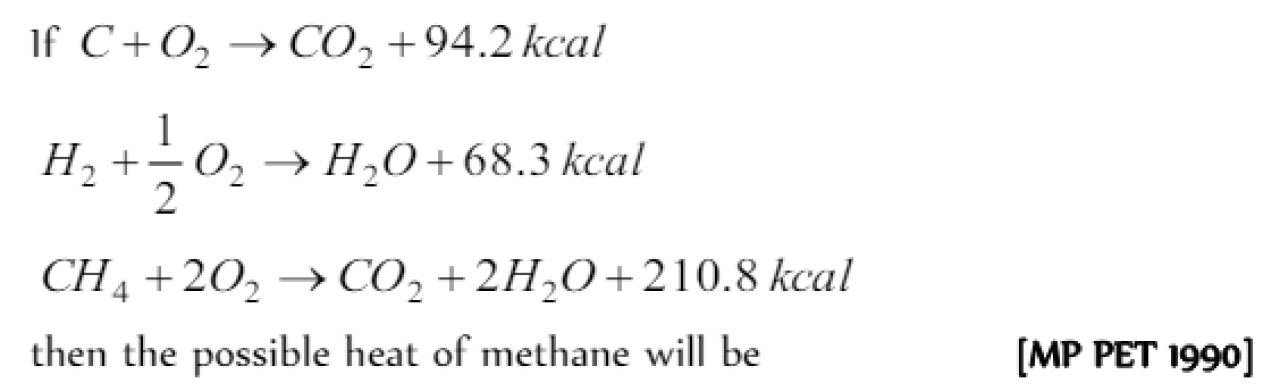Question#1

Question#2
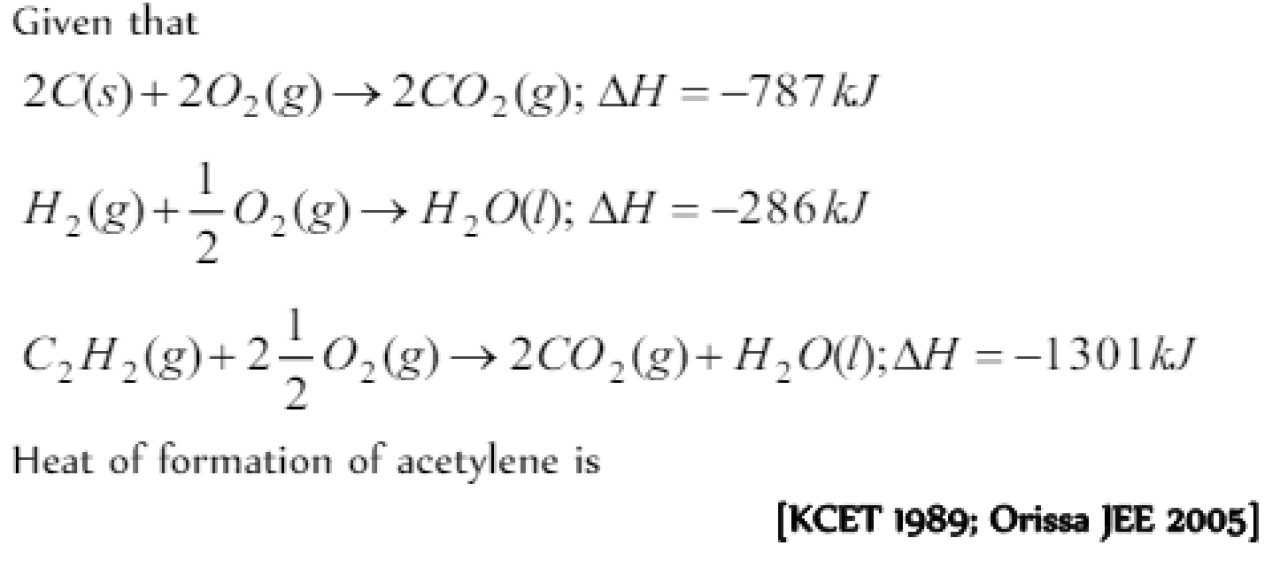
Question#3
Question#4
Question#5
The enthalpy of combustion of methane at 25oC is 890 kJ . The heat liberated when 3.2 g of methane is burnt in air is [KCET 2004]
Question#6
The enthalpies of combustion of carbon and carbon monodie are – 393.5 and –283 kJ mol–1 respectivley. The enthalpy of formation of carbon monoxide per mole is [AIEEE 2004]
Question#7
Which of the following pairs has heat of neutralisation equal to 13.7 Kcals [DCE 2003]
Question#8
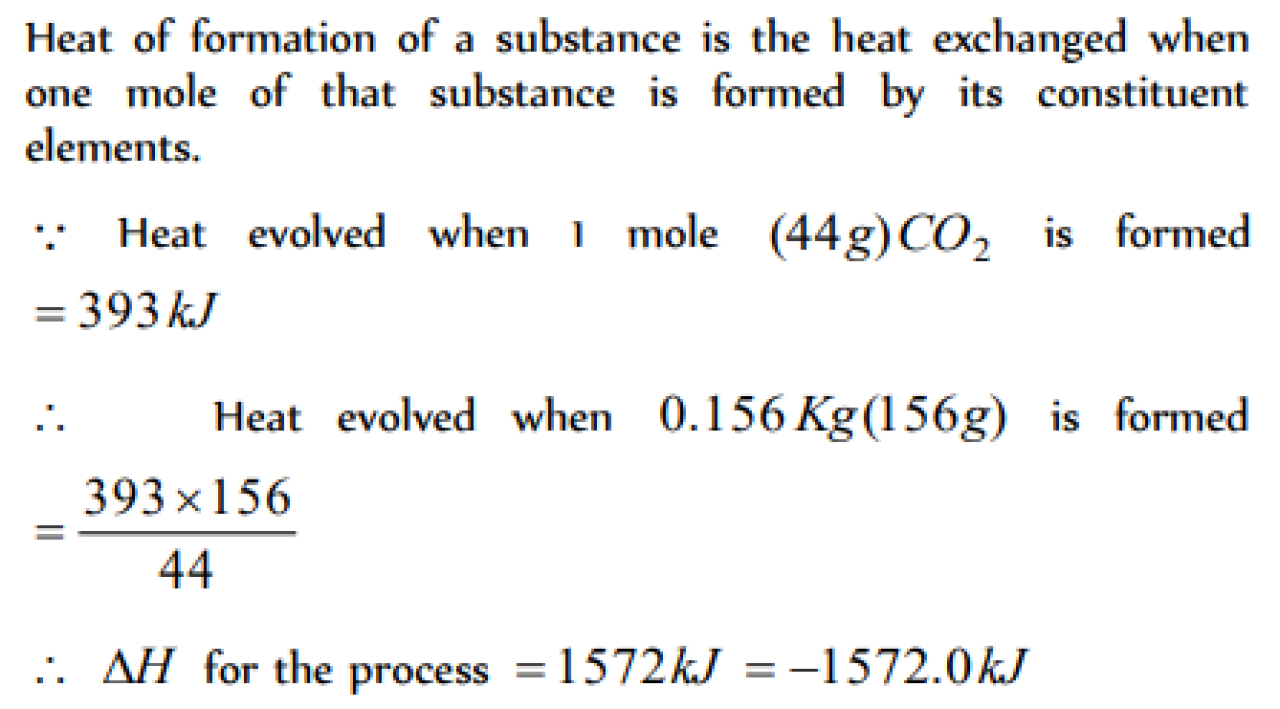
If the heat of formation of CO2 is -393 kJ . The amount of heat evolved in the formation of 0.156 kg of CO2 is [MH CET 2004]
Question#9
Question#10
The heat of combustion of carbon to CO2 is -393.5 kJ / mol . The heat released upon formation of 35.2 g of CO2 from carbon and oxygen gas is [UPSEAT 2004]
Question#11
For complete neutralization of HCl with NaOH, the heat of neutralization is [MP PET 2003]
Question#12
What is the weight of oxygen that is required for the complete combustion of 2.8 kg of ethylene? [BVP 2003]
Question#13
Question#14
Enthalpy change for a reaction does not depend upon [AIEEE 2003]
Question#15
Question#16
One gram sample of NH4 NO3 is decomposed in a bomb calorimeter. The temperature of the calorimeter increases by 6.12 K the heat capacity of the system is 1.23 kJ/g/deg. What is the molar heat of decomposition for NH4 NO3 [AIIMS 2003]
Question#17
Question#18
1 mole of conc. HCl requires X moles of dilute NaOH for neutralisation and 1 mole of concentrate H2SO4 requires Y moles of small dilute NaOH then which of the following reaction is true [MH CET 2001]
Question#19
On combustion, carbon forms two oxides CO and CO2, Heat of formation of CO2 gas is 94.3 kcal and that of CO is 26.0 kcal. Heat of combustion of carbon is [JIPMER 2002]
Question#20
Which of the following reactions is not exothermic [MP PET 2002]
Question#21
If a mole of H2 molecule is heated to high temperature the following reaction takes place [Kerala (Med.) 2002]
Question#22
The heat of neutralisation will be highest in [MP PMT 2002]
Question#23
Enthalpy of neutralisation of NH4OH and HCl , is numerically [JIPMER 2002; Kurukshetra CEE 2002]
Question#24
Question#25
Question#26
Question#27
Compounds with high heat of formation are less stable because [KCET 2002]
Question#28
Question#29
When the aqueous solution of 0.5 mole HNO3 is mixed with the 0.3 mole of OH- solution, then what will be the liberated heat (Enthalpy of neutralization is = 57.1 kJ) [Kerala CET 2005]
Question#30
Question#31
A system is changed from state A to state B by one path and from B to A another path. If E1 and E2 are the corresponding changes in internal energy, then [Pb. PMT 2001]
Question#32
Heat of neutralization of strong acid and weak base is [UPSEAT 2001]
Question#33
Question#34
Question#35
In order to decompose 9 g water 142.5 kJ heat is required. Hence the enthalpy of formation of water is [KCET 2001]
Question#36
For exothermic reaction, the equilibrium constant [JIPMER 2001]
Question#37
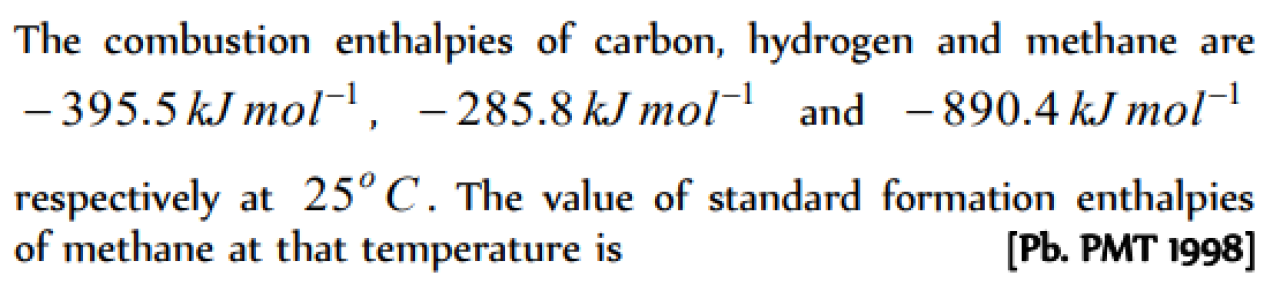
The values of 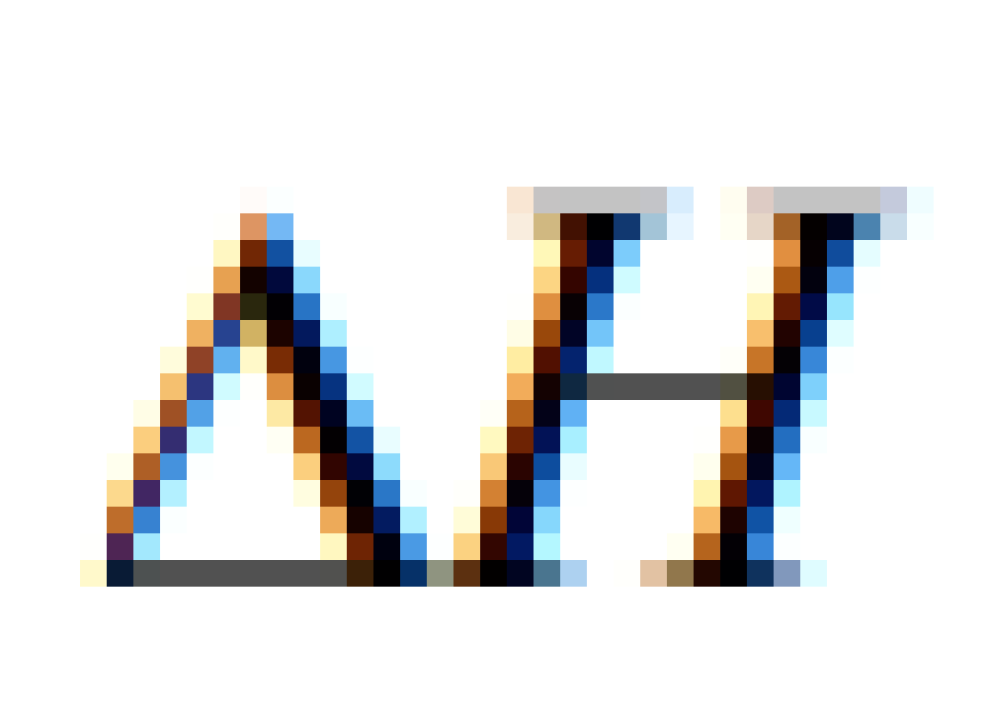
Question#38
Question#39
If the heat of combustion of carbon monoxide at constant volume and at 17oC is – 283.3 kJ, then its heat of combustion at constant pressure ( R= 8.314 degree-1 Jmol-1) [CPMT 2001]
Question#40
Question#41
The heat of neutralization of HCl and NaOH is [MP PET 2001]
Question#42
Question#43
The enthalpy of combustion of C6H6(l) is – 3250 kJ. When 0.39 g of benzene is burnt excess of oxygen in an open vessel, the amount of heat evolved is [KCET 2000; AFMC 2000; DCE 2000]
Question#44
Question#45
Question#46
Which of the following compounds will absorb the maximum quantity of heat when dissolved in the same amount of water ? The heats of solution of these compounds at 25oC in kJ/mole of each solute is given in brackets [AMU (Engg.) 2000]
Question#47
Question#48
Question#49
Question#50
Question#51
Heat of neutralization of the acid-base reaction is 57.32 kJ for [JIPMER 2000]
Question#52
Question#53
Question#54
2.1 g of Fe combines with S evolving 3.77 kJ. The heat of formation of FeS in kJ/mol is [AMU (Engg.) 1999]
Question#55
When a strong acid, strong base or their salt are dissolved in water, they are completely ionised. If a strong acid is added to a strong base, H+ ions from the former combine with OH- ions of the latter forming water. The formation of each water molecule liberates a certain quantity of energy and the reaction is exothermic. The heat liberated when one mole of water is formed by combining hydrochloric acid and sodium hydroxide is 13.7 kcal. The heat liberated when one mole of water is formed by combining sulphuric acid and sodium hydroxide is [AMU (Engg.) 1999]
Question#56
When 4 g of iron is burnt to ferric oxide at constant pressure, 29.28 kJ of heat is evolved. What is the enthalpy of formation of ferric oxide (At. Wt. of Fe = 56) [AIIMS 1999]
Question#57
The molar enthalpy of vaporisation of water at 1 atm and 25oC is 44 kJ. The standard enthalpy of formation of 1 mole of water vapour at 25oC is
Question#58
When 50 cm3 of a strong acid is added to 50 cm3 of an alkali, the temperature rises by 5oC . If 250 cm3 of each liquid are mixed, the temperature rise would be [KCET 1999]
Question#59
Question#60
Question#61
Question#62
Ozone is prepared by passing silent electric discharge through oxygen. In this reaction [AFMC 1998]
Question#63
When 0.5g of sulphur is burnt to SO2 ,4.6 kJ of heat is liberated. What is the enthalpy of formation of sulphur dioxide [KCET 1998; AFMC 2001]
Question#64
The neutralisation of a strong acid by a strong base liberates an amount of energy per mole of H+ that [BHU 1998]
Question#65
Equal volumes of methanoic acid and sodium hydroxide are mixed. If x is the heat of formation of water, then heat evolved on neutralisation is [BHU 1998]
Question#66
Question#67
Question#68
The enthalpy change of a reaction does not depend on [AIIMS 1997]
Question#69
Question#70
Question#71
8 gm of CH4 is completely burnt in air. The number of moles of water produced are [Orissa JEE 1997]
Question#72
Fermentation is a reaction called [RPMT 1997]
Question#73
Calculate the standard heat of formation of carbon disulphide (l) , given that the standard heat of combustion of carbon (s) , sulphur (s) and carbon disulphide (l) are -393.3,- 293.72 and -1108.76 kJ mol-1 respectively [Roorkee 1989; BHU 1997]
Question#74
Question#75
Question#76
Question#77
Standard molar enthalpy of formation of CO2 is equal to [IIT JEE 1997; BHU 2001]
Question#78
Question#79
2.2016 gm of acetaldehyde produced 13.95kcal of heat on combustion in O2 . Calculate the heat of combustion of CH3CHO [Bihar CEE 1995]
Question#80
Question#81
The heat of reaction at constant pressure is given by [MP PMT 1997]
Question#82
Enthalpy of formation of HF and HCl are -161kJ and -92kJ respectively. Which of the following statements is incorrect [KCET 2003]
Question#83
Question#84
Question#85
Question#86
When water is added to quick lime, the reaction is [MP PMT 1995]
Question#87
Question#88
Question#89
Question#90
Complete combustion of CH 4 gives [BHU 1995]
Question#91
In the combustion of 2.0 gm of methane 25kcal heat is liberated, the heat of combustion of methane would be [MP PMT 1994]
Question#92
The energy evolved is highest for which of the following reactions[MP PET 1994]
Question#93
For an exothermic reaction [MP PET 1994; Manipal MEE 1995]
Question#94
Question#95
Heat of transition is the heat evolved or absorbed when a substance is converted from [KCET 1984]
Question#96
Question#97
Question#98
In which of the following reactions does the heat change represent the heat of formation of water [EAMCET 1991]
Question#99
[IIT JEE (Screening) 1992]
Question#100
Which of the following values of heat of formation indicates that the product is least stable [MP PMT 1991]
Question#101
Question#102
Question#103
Question#104
Question#105
Question#106
The formation of water from H2 (g) and O2(g) is an exothermic reaction because [MP PMT/PET 1988]
Question#107
Question#108
Question#109
Which of the following reactions can be used to define the heat of formation of CO2(g) [MP PMT 1989; MH CET 2001]
Question#110
Question#111
Which of the following statements is correct about heat of combustion [MADT Bihar 1982]
Question#112
One of the phenomena which cannot be described as combustion is [EAMCET 1979]
Question#113
Which of the following is an endothermic reaction [EAMCET 1980; MP PMT 1980; IIT JEE 1989; JIPMER 2002]
Question#114
A reaction that takes place with the absorption of energy is [EAMCET 1977]
Question#115
If a chemical reaction is accompanied by the evolution of heat, it is [BHU 1979]
Question#116
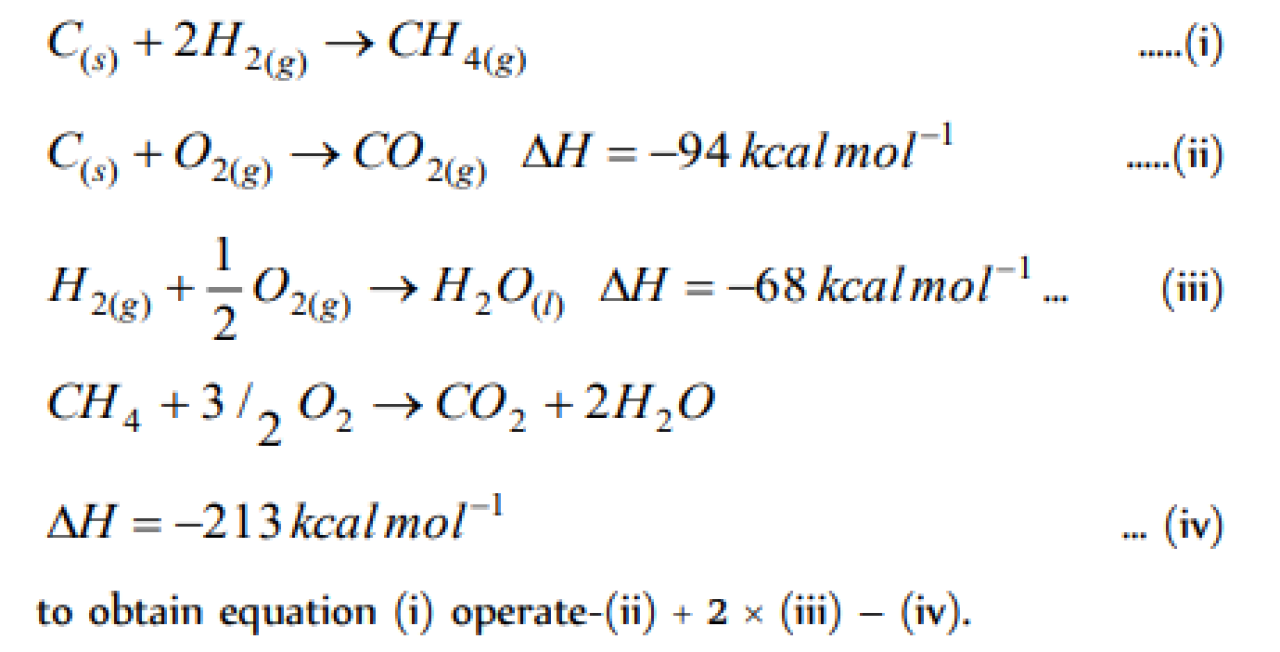
The heat of combustion of CH4(g), C (graphite) , and H2(g) is 20kcal,-40kcal and -10kcal respectively. The heat of formation of methane is [EAMCET 1998]
Question#117
Question#118
All reactions with chemical dissociation are [MP PMT 1990]
Question#119
Question#120
Question#121
The lowest value of heat of neutralization is obtained for [KCET 1988; MP PMT 1990]
Question#122
Question#123
The compound with negative heat of formation are known as[DPMT 1981]
Question#124
Which is the best definition of “heat of neutralization” [CMC Vellore 1991]
Question#125
Enthalpy of a compound is equal to its [CMC Vellore 1991]
Question#126
A solution of 500ml of 0.2 M KOH and 500ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1 . The experiment is repeated using 250ml each of solution, the temperature raised is T2 . Which of the following is true [EAMCET 1987; MP PET 1994]
Question#127
The heat of neutralisation of a strong acid and a strong alkali is 57.0 kJ mol-1 . The heat released when 0.5mole of HNO3 solution is mixed with 0.2 mole of KOH is [KCET 1991; AIIMS 2002; AMU (Engg.) 2002]
Question#128
The heat of combustion of carbon is –94 kcal at 1 atm pressure. The intrinsic energy of CO2 is
Question#129
Which of the following fuels will have the highest calorific value (kJ/kg) [MP PMT 1990]
Question#130
The heat of formation of H2O (l) is – 68.0 kcal, the heat of formation of H2O (g) is likely to be
Question#131
The heats of combustion of rhombic and monoclinic sulphur are respectively 70960 and 71030 calories. What will be the heat of conversion of rhombic sulphur to monoclinic [MP PMT/PET 1988]
Question#132
Question#133
Question#134
Heat of combustion of a substance [CPMT 1987, 96; AFMC 1992]
Question#135
Question#136
Which of the following reaction is endothermic [AFMC 1988]
Question#137
. The standard molar heat of formation of ethane, CO2 and water (l) are respectively – 21.1, – 94.1 and – 68.3 kcal. The standard molar heat of combustion of ethane will be [IIT JEE 1986; DPMT 2005]
Question#138
Heat of neutralisation of an acid by a base is highest when [KCET 1985]
Question#139
Heat of neutralisation of a strong acid by a strong base is a constant value because [KCET 1984]
Question#140
The heat of reaction does not depend upon
Question#141
Heat of formation of CO2(g) HO2(l) and CH4(g) are – 94.0, – 68.4, and – 17.9 kcal respectively. The heat of combustion of methane is
Question#142
Question#143
Heat of neutralisation of NH4OH and HCl is [EAMCET 1980; Roorkee 1990; MP PMT 1994]
Question#144
The enthalpy of combustion at 25°C of H2 , cyclohexane (C6H12) and cyclohexene (C6H10) are –241, –3920 and –800 KJ / mole respectively. The heat of hydrogenation of cyclohexene is [BHU 2005]
Question#145
The heat of formations of CO(g) and ( ) CO2 g are -26.4 kcal and -94.0 kcal respectively. The heat of combustion of carbon monoxide will be [MP PET/PMT 1988; EAMCET 1993]
Question#146
The mutual heat of neutralization of 40 gm of NaOH and 60 gm CH3COOH will be [MP PET/PMT 1988]
Question#147
Question#148
The enthalpy of neutralization is about 57.3 kJ for the pair
Question#149
Question#150
Question#151
If the enthalpy of B is greater than of A, the reaction A -> B is [MP PMT 1997]
Question#152
Which of the following is an example of endothermic reaction[MP PMT 1980]
Question#153
Question#154
Question#155
Question#156
Question#157
Question#158
Which of the following statement is correct [NCERT 1978]
Question#159
An endothermic reaction is one in which [MNR 1980; NCERT 1976]
Question#160
An exothermic reaction is one which [NCERT 1977; MP PMT 1990]
Question#161
Evaporation of water is [CPMT 1973; DPMT 1982; MP PMT 1989; MP PET 1999]
Question#162
The following is (are) endothermic reaction [IIT JEE 1999]
Question#163
Question#164
Question#165
An exothermic reaction is one in which the reacting substances[CPMT 1974, 79; Bihar MEE 1982; KCET 1992; JIPMER 2001]
Question#166
The molar neutralization heat for KOH and HNO3 as compared to molar neutralization heat of NaOH and HCl [MP PMT 1989]
Question#167
From Kirchhoff's equation which factor affects the heat of reaction[MP PMT 1990]
Question#168
Question#169
In which of the following neutralisation reactions, the heat of neutralisation will be highest [MP PMT 1989, 91; AIIMS 1999]
Question#170
The enthalpy of fusion of ice per mole
Question#171
Question#172
Question#173
What is the reason of energy change in a chemical reaction? MDCAT2023
Question#174
\( \frac{1}{2} H_2 \rightarrow H \ \Delta H =+218kJmol^{-1} \)
In this reaction \( \Delta H \) will be called MDCAT2016
Question#175
The equation represents standard enthalpy of atomization of hydrogen is MDCAT2015
Question#176
Heat of formation( \( \Delta H_f \) )for CO2 is MDCAT2013
Question#177
The absolute enthalphy of neutralisation of the reaction
MgO(s) + HCl(aq) → MgCl(aq) + H2O(l) will be
[CBSE PMT 2005]
Question#178
The following is (are) endothermic reaction [IIT JEE 1999]
Question#179
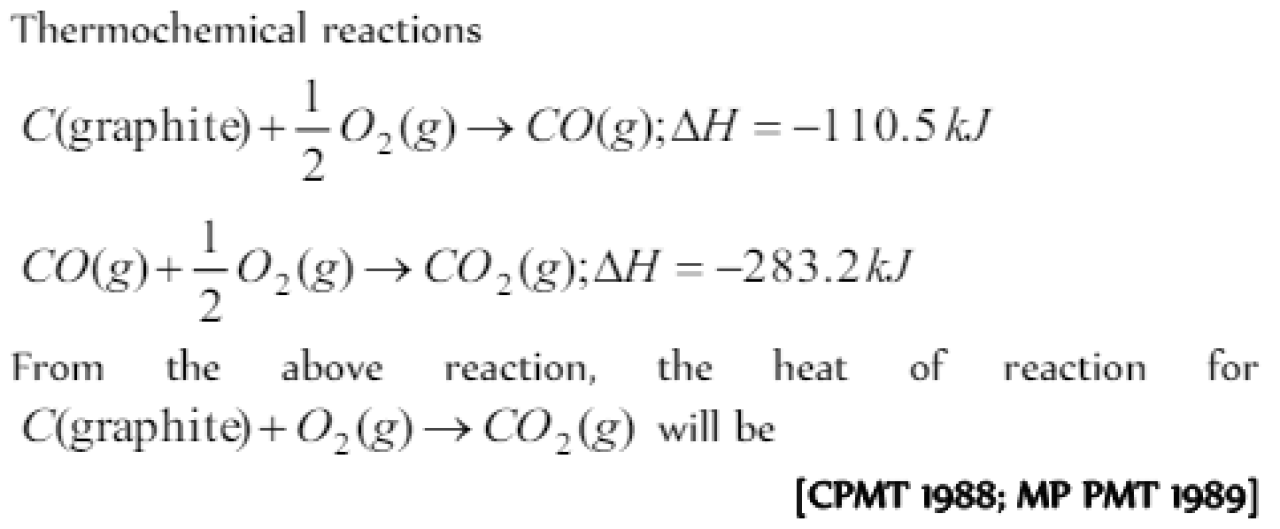
Thermochemical reactions
C (graphite) + 1/2O2 →CO(g) ΔH= -110.5 KJ
CO(g) +1/2 O2(g) →CO2(g); ΔH=-283.2 kJ
From the above reaction, the heat of reaction for
C (graphite) + O2 (g) →CO2(g) will be [CPMT 1988; MP PMT 1989]
Question#180
The molar neutralization heat for KOH and HNO3 as compared to molar neutralization heat of NaOH and HCl
[MP PMT 1989]
Question#181
In which of the following neutralisation reactions, the heat of
neutralisation will be highest
[MP PMT 1989, 91; AIIMS 1999]