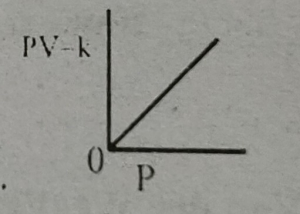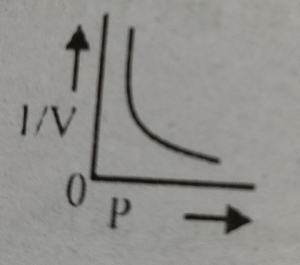Question#1
A closed vessel contains equal number of nitrogen and oxygen molecules at a pressure of P mm. If nitrogen is removed from the system then the pressure will be [MP PMT 1985]
Question#2
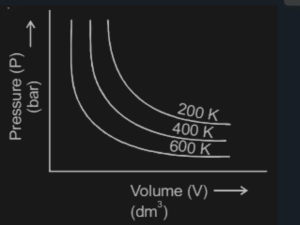
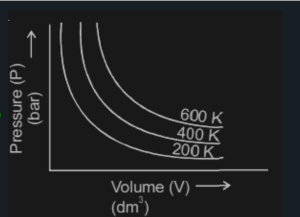
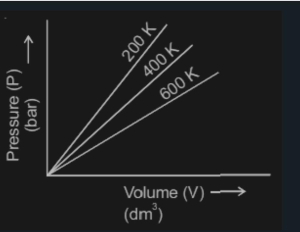
Choose the correct option for graphical representation of Boyle;s law, which shows a graph of pressure vs volume of a gas at different temperatures NEET2021
a) | b) |
c) | d) |
Question#3
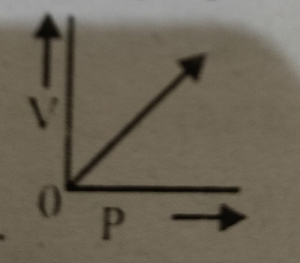
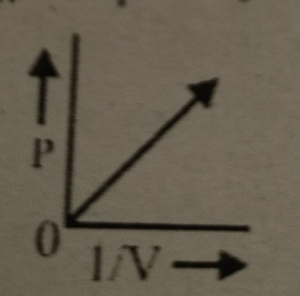
Which graph represents Boyle's Law MDCAT-2015
a) | b) |
c) | d) |
Question#4
Which graph represents Boyle's law
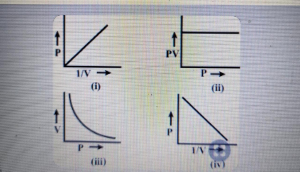
Question#5
At constant temperature if the pressure of gas is doubled its volume becomes [ETEA2010] [ETEA-2011 Med][ETEA-2007 Med]
Question#6
Which of the following statement is false [BHU 1994]
Question#7
If 20cm3 gas at 1 atm. is expanded to 50 cm3 at constant T, then
what is the final pressure [CPMT 1988]
Question#8
Air at sea level is dense. This is a practical application of
[Kerala CEE 2000]
Question#9
N2 is found in a litre flask under 100kPa pressure and O2 is found in another 3 litre flask under 320 kPa pressure. If the two flasks are connected, the resultant pressures is [Kerala PMT 2004]
P = 100 kPa , P1 =? , V = 1 litre , V1=4litre
applying Boyle's law PV = P1V1
100 x 1 = P1 x 4 ;
P1 = 25
If P2 is the pressure of O2 gas in the mixture of O2 and N2 then, 320 x 3 = P2 x 4 ;
P2 =240
Hence, Total pressure P = P1 + P2 = 25 + 240 =265 kPa